Signalment:
Gross Description:
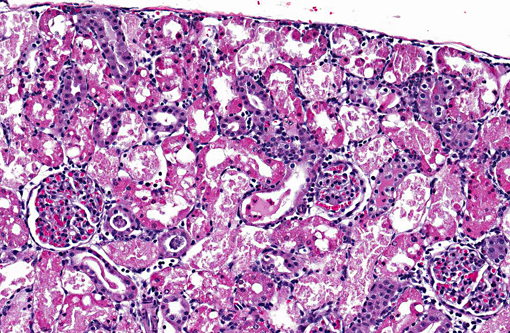
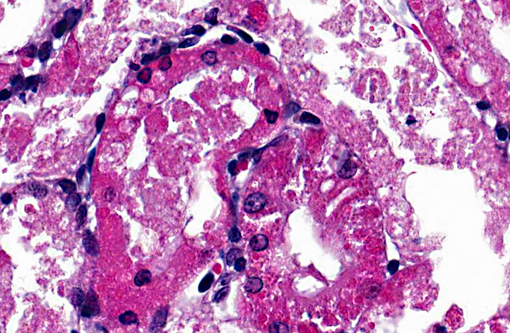
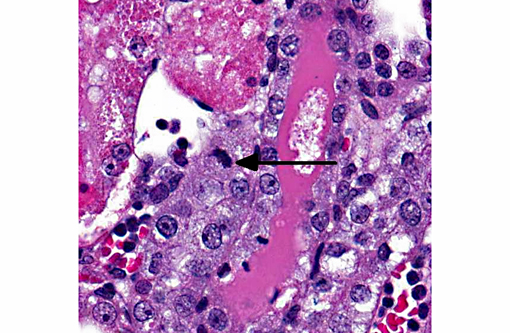
Histopathologic Description:
Morphologic Diagnosis:
1. Acute diffuse degeneration and necrosis of the cortical proximal tubules with cytoplasmic hyaline droplet formation.
2. Cortical interstitial cell proliferation with mild lymphohistiocytic interstitial nephritis.
Lab Results:
| Serum chemistry | Normal | Day 4 | Day 5 |
| BUN, mg/dL | 7 - 27 | 30 | 103 |
| Creatinine, mg/dL | 0.4 1.8 | 0.8 | 3.0 |
| Phosphorus, mg/dL | 2.1 6.3 | 9.5 | 13.0 |
| Urinalysis | Day 4 | Day 5 | |
| Specific gravity | 1.010 | 1.016 | |
| Protein, mg/dL | <100 | 300-500 | |
Condition:
Contributor Comment:
There are several hypotheses as to the mechanism of PCT cell necrosis in gentamicin-induced nephrotoxicity. Gentamicin changes lysosomal membrane permeability which may result in lysosomal enzyme leakage and necrosis.(9) There are several examples of PCT lysosomal overload resulting in toxicity such as in the unique male rat α2u-globulin nephropathy, Bence-Jones nephropathy and nitrilotriacetic acid nephrotoxicity. It has been suggested that gentamicin is toxic through non-lysosomal targets including disruption of ion transport and enzyme release at the apical and basolateral membranes.(9) Recent studies have indicated free radicals are important mediators of damage in gentamicin nephrotoxicity. Gentamicin has been shown to enhance the generation of superoxide anion and hydrogen peroxide by mitochondria. Gentamicin has also been shown to lead to the release of iron from renal cortical mitochondria and to enhance generation of hydroxyl radicals which are produced by the superoxide anion and hydrogen peroxide in the presence of a metal catalyst, in this case iron. Free radical scavengers and metal chelators have been shown to ameliorate gentamicin-induced renal injury.(1)
On gross examination the kidneys may be pale and enlarged. Microscopically, acute tubular necrosis is restricted to the S1 and S2 segments (pars convoluta) of the PCT, though hyaline droplets are prominent throughout all segments of the proximal tubule.(9) Clinically gentamicin toxicity results in the inability to concentrate urine, polyuria, glucosuria, proteinuria, phospholipiduria and increased serum BUN and creatinine.8 Dysfunction of the PCT results in decreased uptake of glucose, organic bases and low molecular weight protein resulting in their excretion in the urine. Phospholipiduria results in phospholipid-rich myeloid bodies that are shed from the damaged microvillar surfaces of the PCT and can be seen in the urine sediment.(3)
Proximal tubule intracytoplasmic hyaline droplet formation may be associated with accumulation of α2u-globulin. This is a gender specific phenomenon and is only seen in mature male rats. These hyaline droplets represent an accumulation of secondary lysosomes containing α2u-globulin and/or albumin within the cytoplasm. Under toxicologic and pathologic conditions hyaline droplets may also consist of other proteins (e.g. histiocytic sarcoma associated hyaline droplets contain lysozyme).(6) Hyaline droplet formation is thought to occur through two mechanisms: increased endogenous protein production or interference with lysosomal enzyme-mediated hydrolysis occurring in the renal epithelial cell. It has been reported that lysosomal bodies in the PCT of rats autofluoresce5; the hyaline droplets in the PCT of this rat demonstrate this phenomenon. A Mallorys Heidenhain or chromotrope-aniline-blue stain is used to visualize the α2u-globulin component and PAS is used to demonstrate the albumin component of the hyaline droplets in this case.(4,5,7) As a highly exaggerated dose of gentamicin was used in this study to enable a rapid development of necrosis, specific evidence of phospholipidosis in H&E section are not apparent in this case. However, a Bakers stain in frozen section or a toluidine blue stain of plastic section could be anticipated to dramatize the phospholipidotic accumulations and debris.
Gentamicin nephrotoxicity can be reversible. Regeneration of PCT epithelium progresses even with continued dosing of gentamicin. It is thought that regenerating epithelium is more resistant to toxicity than mature PCT epithelium.(8) However, over chronic time frames in toxic exposures, even at doses that do not cause necrosis, increased cell turnover can be demonstrated and exacerbation of lesions of chronic progressive nephropathy may be the longer term consequence.
JPC Diagnosis:
Conference Comment:
Because of their unique mechanisms of toxicity, different drugs affect different specific segments of the nephron, which may assist in narrowing the differential diagnosis in a diagnostic setting. The proximal convoluted tubule is the most commonly-affected segment; numerous drugs cause injury here, including gentamicin, cyclosporine, and cisplatin. The distal tubules are damaged by cyclosporine, sulfadiazine, and amphotericin B, among others. The collecting duct is affected by amphotericin B and acyclovir, and the loop of Henle can be damaged by chronic exposure to analgesics. Glomerular damage can be caused by doxorubicin, gold and penicillamine.(2)
Conference participants discussed serum biomarkers of renal injury such as serum creatinine and blood urea nitrogen, which despite lacking sensitivity, are commonly used to detect renal damage, and are elevated in severe renal disease, as in this case. Serum creatinine, the standard biomarker for glomerular filtration rate, increases only after substantial injury, and is affected by both systemic production and tubular secretion of creatinine. Blood urea nitrogen is also used to measure renal function, but it can be affected by many factors, including urea production by the liver; in addition to being filtered by the glomerulus, it is also reabsorbed by other parts of the nephron.(2) For these reasons, recent research through the Predictive Safety Testing Consortium has been aimed at identifying second-generation biomarkers for acute kidney injury in both laboratory animal models and humans. The goal is to identify markers for early detection of renal injury as well as localization of the injury. Although none have yet shown sufficient specificity and sensitivity for clinical use, several markers have been proposed, including the following:(2)
- Kidney injury molecule-1 (KIM-1), interleukin-18 (IL-18), and fatty acid binding protein-liver type (L-FABP) which are increased with damage to proximal tubules
- Neutrophil gelatinase-associated lipocalin (NGAL) and clusterin, which are associated with damage to proximal and distal tubules
- Osteopontin, which is associated with damage to proximal tubules, loops of Henle and distal tubules
- Cystatin C, which is associated with damage to glomeruli and proximal tubules
Histopathology remains the gold standard for identifying renal injury; however, these or other biomarkers may prove to reliably detect kidney injury in both experimental animals and in a clinical setting.(2)
References:
2. Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol. 2010;28:436-440.
3. Cheville N. Ultrastructural Pathology: The Comparative Cellular Basis of Disease. 2nd ed. Ames, Iowa: Wiley-Blackwell; 2009.
4. de Rijk EP, Ravesloot WT, Wijnands Y, van Esch E. A fast histochemical staining method to identify hyaline droplets in the rat kidney. Toxicol Pathol. 2003;31:462-464.
5. Hard GC. Some aids to histological recognition of hyaline droplet nephropathy in ninety-day toxicity studies. Toxicol Pathol. 2008;36:1014-1017.
6. Hard GC, Snowden RT. Hyaline droplet accumulation in rodent kidney proximal tubules: an association with histiocytic sarcoma. Toxicol Pathol. 1991;19:88-97.
7. Haschek WM, RC, Wallig WA. Handbook of Toxicologic Pathology, Two-Volume Set, 2 ed. San Diego, CA: Academic Press; 2002.
8. Maxie MG. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Philadelphia, PA: Elsevier Saunders; 2007.
9. Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43:1003-1012.
10. Newman SJ. The urinary system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby; 2012:602-603.