Wednesday Slide Conference, Conference 21, Case 1
Signalment:
9-month-old female Sprague Dawley rat (Rattus norvegicus), SRG genotype (Rag2 & IL-2Rγ knockout)
History:
This rat was part of a cancer implantation study using a novel cell line. Cancer cells were implanted around 3-months-old but failed to grow. The rat was monitored for delayed growth for an additional 6 months until its presentation to clinical staff. Weekly monitoring of this rat identified a drop in weight which persisted over a 3 week span despite clinical interventions, and the rat was ultimately euthanized.
Gross Pathology:
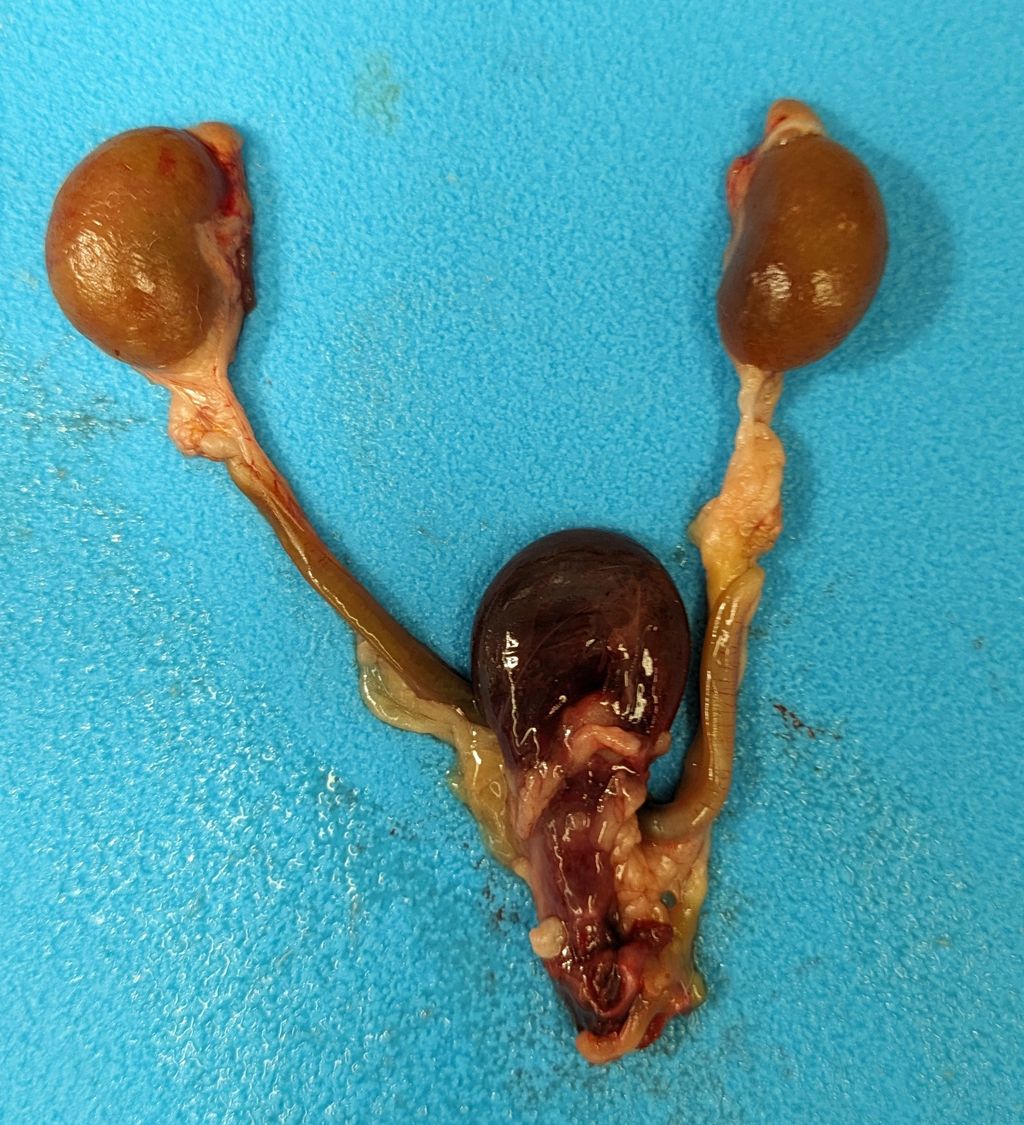
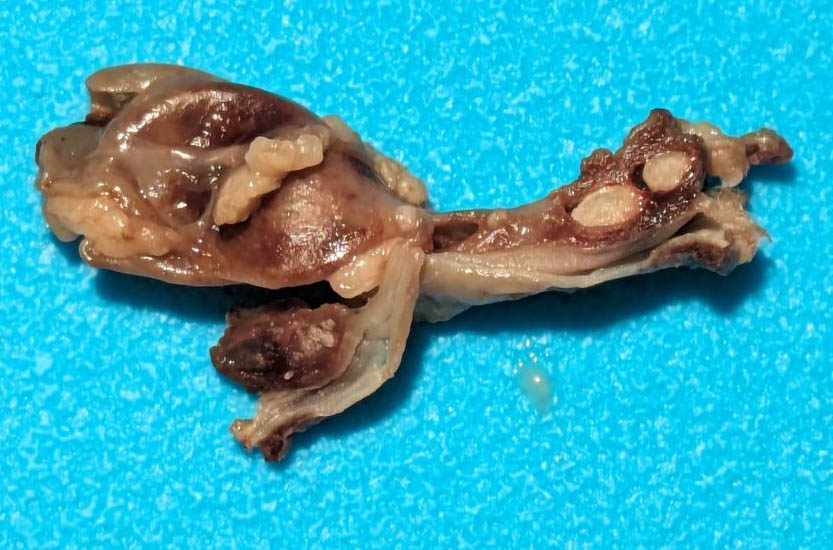
The urinary bladder was markedly distended by large amounts of red-tinged fluid, with blood-clots both suspended and adherent to the mucosal surface. The mucosal surface was red and roughened. Transmurally the bladder wall was red to black, and this change extended distally through the trigone and throughout the length of the urethra. At the distal end of the urethra, just proximal to the external genitalia were two large (3-5mm diameter), round, smooth, hard calculi (stones) occluding the lumen of the urethra. The stones were embedded within the mucosa and did not excise easily. Both ureters were distended with fluid (hydroureter). Both kidneys were moderately pale tan and had
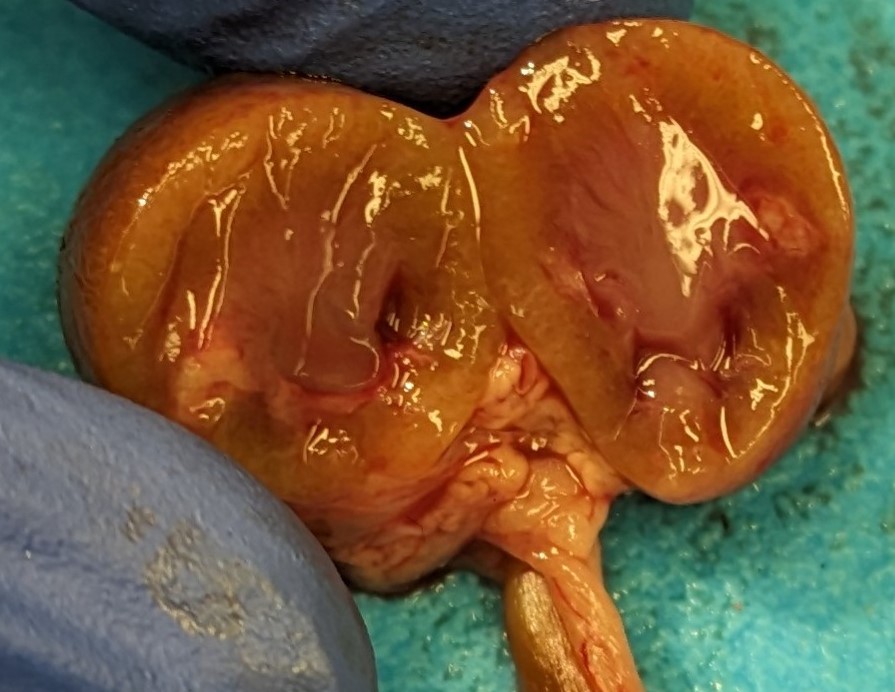
mildly bosselated subcapsular surfaces. There was mild to moderate dilation of the renal pelvis of both kidneys (hydronephrosis). From the renal pelvis, extending proximally through the renal papilla, medulla, and cortex is an irregular, friable, focal region of pallor.
Laboratory Results:
Bacteria Culture & Sensitivity: Tissue (kidney)
- Staphylococcus xylosus - Numerous
- Enterobacter cloacae complex - Few
Pneumocystis spp. Polymerase chain reaction (PCR): Tissue (lung)
- Negative
Microscopic Description:
Cytology (DifQuick):
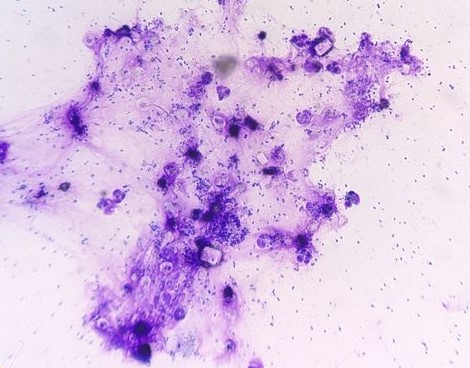
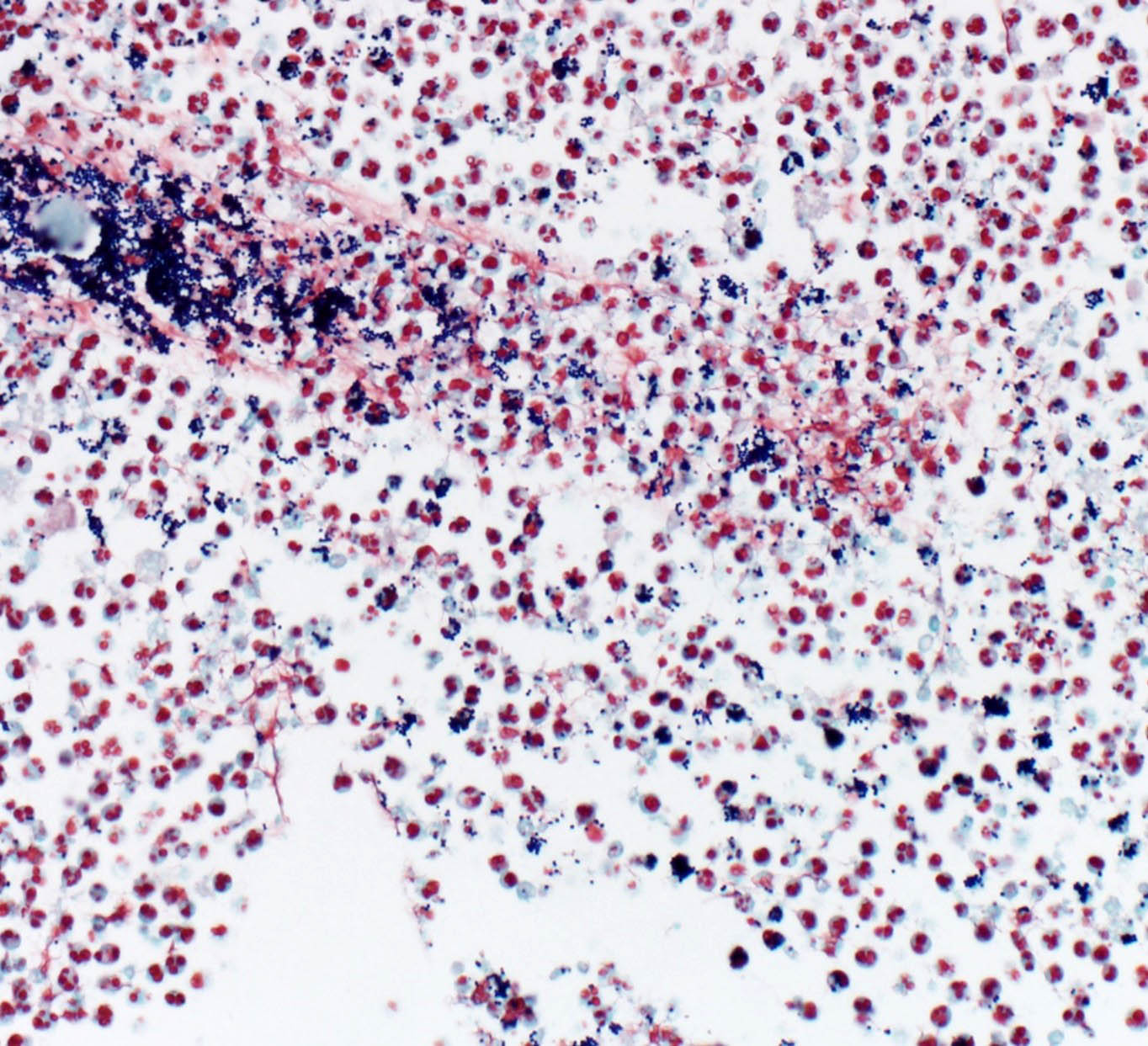
Urine – Cytological preps of urine collected during necropsy showed large numbers of viable and degenerate neutrophils, streaming cellular debris, large numbers of bacterial cocci, and crystals. Bacteria are occasionally observed within neutrophils. Crystals are colorless and rhomboid (presumed struvite).
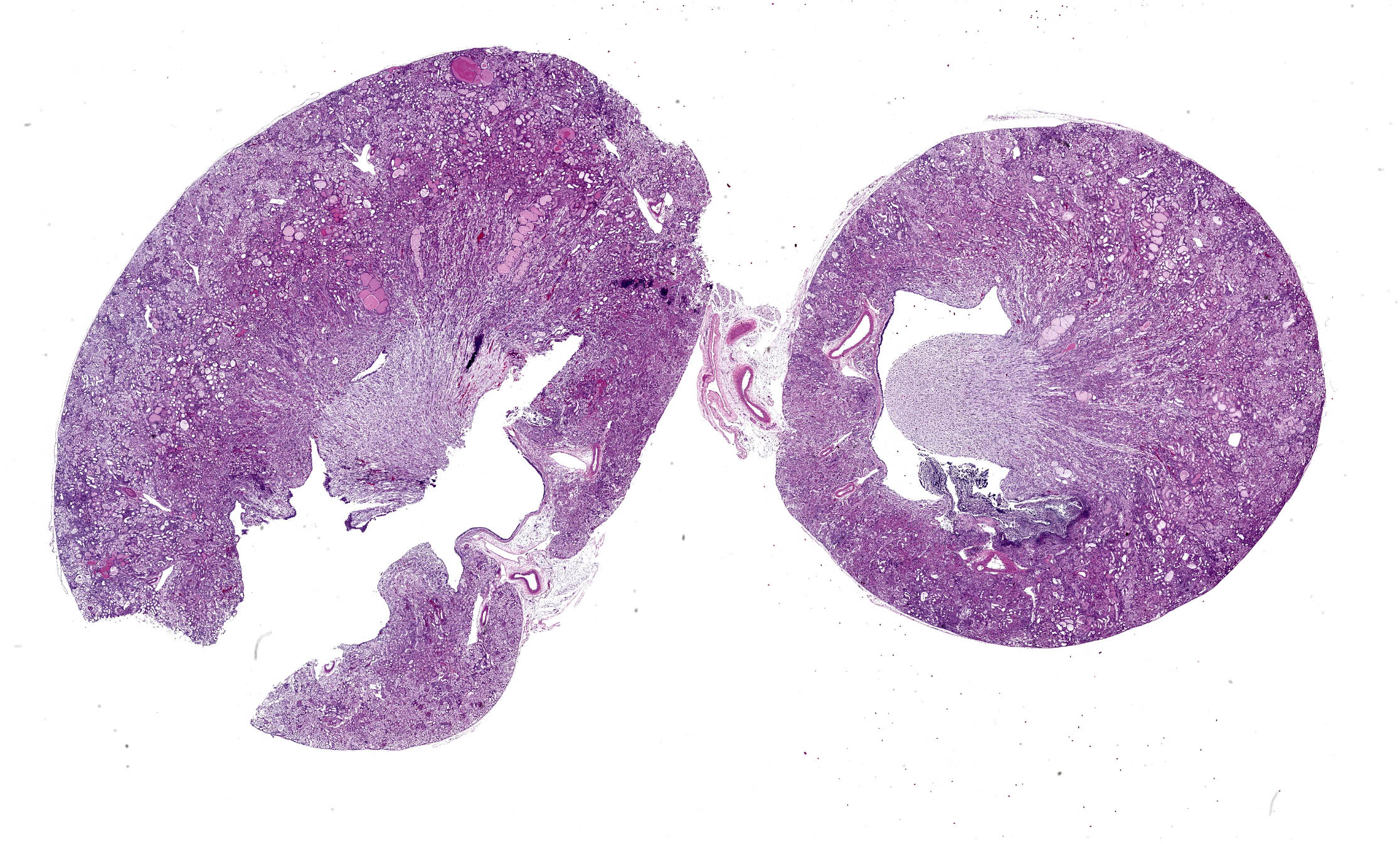
Histology (H&E):
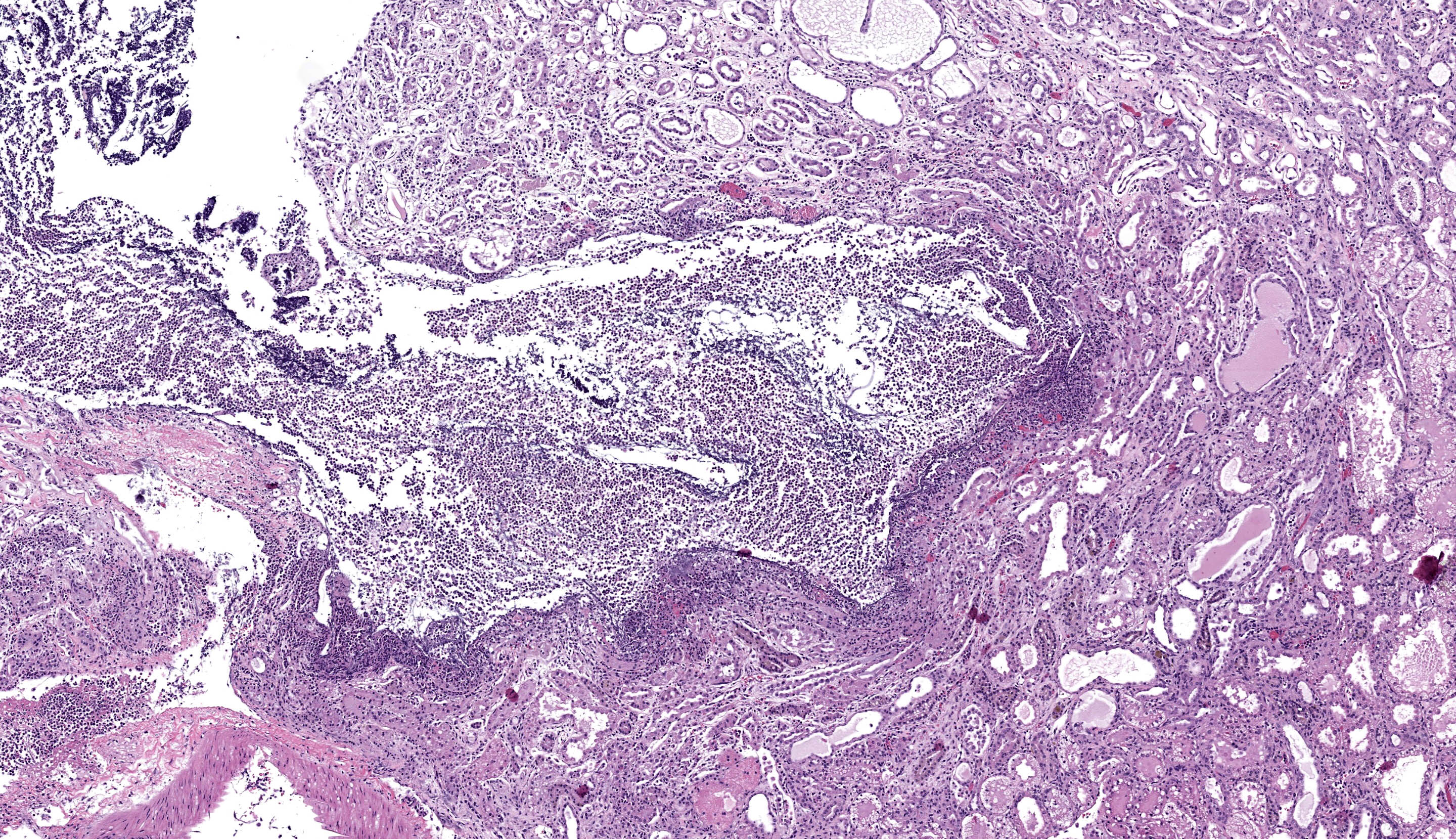
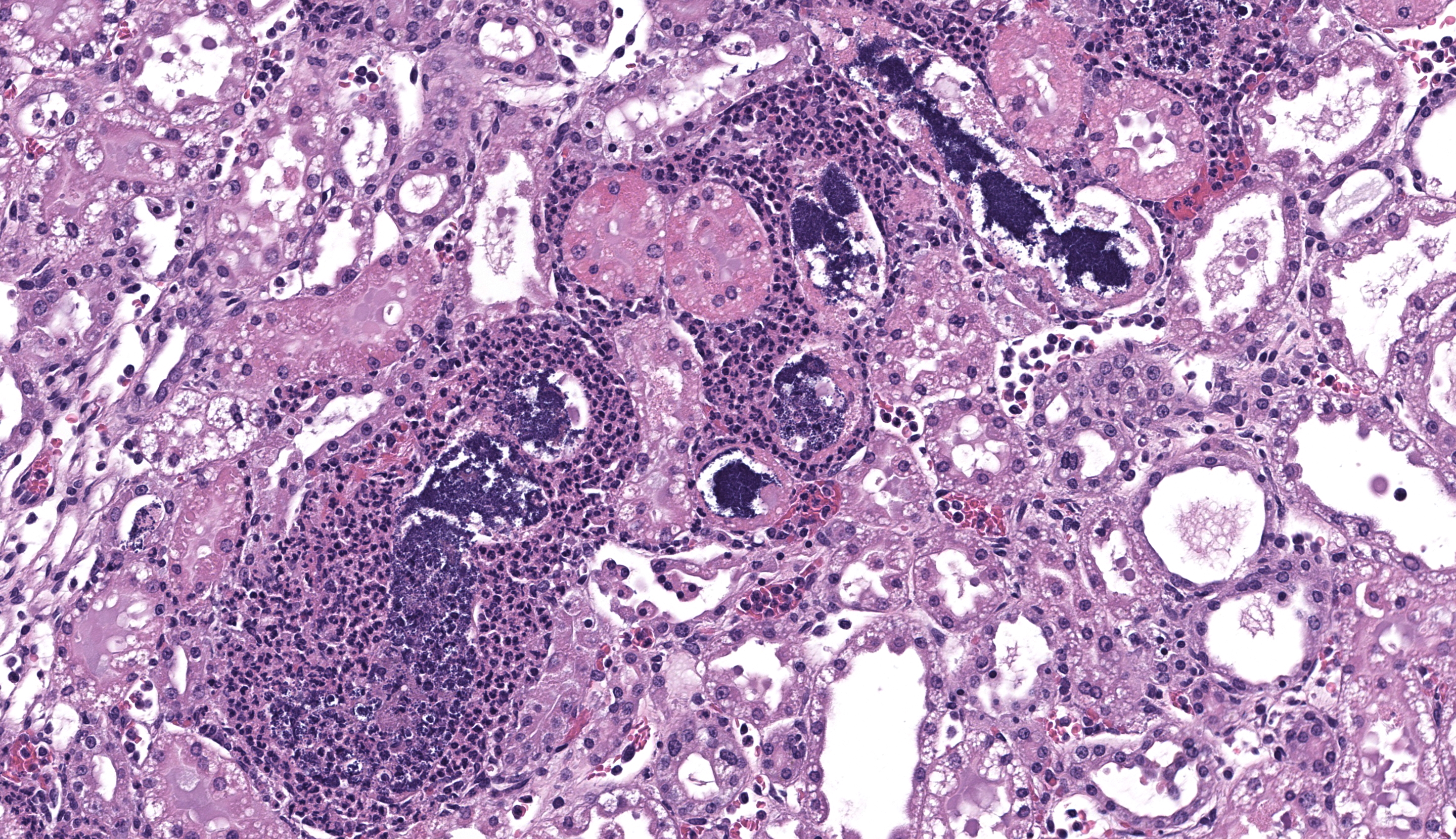
Kidney - Extending from the renal pelvis into the medulla is a regionally extensive area of necrosis and marked suppurative inflammation with large numbers of cocci bacteria admixed throughout the cellular debris and neutrophils (pyelonephritis). The renal parenchyma in the vicinity of this lesion is characterized by interstitial edema, tubular degeneration and necrosis, with tubule lumina often containing eosinophilic proteinaceous fluid, neutrophils, cellular debris, and clusters of bacteria.
In addition to the pyelonephritis described above, there is mild bosselation of the capsular surface of the renal cortex. The cortical and medullary interstitium is expanded by extensive fibrosis with low numbers of mixed mononuclear inflammatory cells. Interstitial fibrosis often replaces tubular profiles. Remaining tubules frequently have markedly dilated lumens which range from empty to variably filled with eosinophilic proteinaceous fluid, fibrillar hyaline casts, or deeply basophilic granular material (mineralization). Tubular epithelial cells range from flattened / attenuated (atrophy) to swollen and vacuolated (degeneration) or are brightly eosinophilic, faded (lysis), and/or sloughed (necrosis). Glomeruli are frequently affected by one or more of the following changes: Dilation of the urinary space, marked global fibrosis of glomerular tufts (glomerulosclerosis), obsolescence of glomerular tufts, adherence of tufts to Bowman’s capsule (synechiae), thickening of Bowman’s capsule basement membrane.
Contributor’s Morphologic Diagnosis:
- Kidney: Pyelonephritis, severe, acute, necro-suppurative, with intralesional cocci bacteria (Gram positive)
- Kidney: Nephropathy, chronic, severe, with multifocal global glomerulosclerosis, periglomerular to interstitial fibrosis, tubular degeneration/necrosis with ectasia and proteinosis, and mild interstitial lymphohistiocytic nephritis (Chronic Progressive Nephropathy)
Contributor’s Comment:
This case highlights the importance of using a range of diagnostic techniques—including cytology, special stains, and culture—alongside standard necropsy and histology to fully understand pathological processes. It serves as a valuable reminder to students to utilize all available tools rather than relying too heavily on one method. The gross identification of pyelonephritis and uroliths led to cytological identification of crystalluria consistent with struvite (triple phosphate/magnesium phosphate) crystals, which suggests the presence of urease producing bacteria. Common urease-producing bacteria associated with struvite crystalluria include: Proteus spp. (gram-negative bacillus), Klebsiella spp. (gram-negative bacillus), Mycoplasma spp. (gram-equivocal, pleomorphic), Corynebacterium urealyticum (gram-positive bacillus), and Staphylococcus spp. (gram-positive cocci). Histological evaluation and Gram staining highlighted clusters of gram-positive cocci, suggesting Staphylococcus spp. as the most likely agent from our differential list of urease-producing bacteria. Lastly, bacterial cultures confirmed Staphylococcus (specifically S. xylosus) as the gram-positive cocci bacteria observed.
In laboratory mice and rats, pyelonephritis is most commonly caused by ascending infections from commensal or gastrointestinal bacteria.3 Urolithiasis is generally considered uncommon, but in cases of pyelonephritis becomes more common when the bacterial culprit is one of those mentioned previously (urease-producing). Staphylococcus xylosus, isolated in this case, is both a common commensal organism of rodents and is urease-producing.3,5,7
Opportunistic infections from commensal organisms such as S. xylosus are likely exacerbated by immunodeficiencies/modulation. In the case of laboratory animals, cystitis and urolithiasis related to S. xylosus has been described in nude mice7 and severe dermatitis has been described in Rag2 / IL-2Rγ knockout strains of mice1 which are the same genes knocked out in the SRG strain rat utilized in this study.
The second morphological diagnosis proposed in this case is consistent with Chronic Progressive Nephropathy (CPN). This condition is one of the most well-described lesions in laboratory rats.3,5 In particular, CPN is exceptionally common in Sprague-Dawley and Fischer 344 strains, with many rats developing clinically significant changes by 8-10 months old, and some developing histologic changes as early as 2-3 months.5 While CPN is generally thought of as a spontaneous or age related disease, it has been recognized that high-protein diets worsen the disease severity.3,5 Despite some persistent ambiguities in the pathogenesis of the condition, any resident or pathologist working in laboratory settings quickly becomes familiar with CPN and the spectrum of lesions it presents with, making it both a “classic” lesion as well as a practically relevant one.
Contributing Institution:
University of Michigan, Unit for Laboratory Animal Medicine, Pathology Core
https://animalcare.umich.edu/business-services/ulam-pathology-core/
JPC Diagnosis:
- Kidney: Tubular degeneration, necrosis, and regeneration, diffuse, moderate, with chronic interstitial lymphoplasmacytic nephritis.
- Kidney: Pyelonephritis, necrotizing and suppurative, subacute, multifocal to coalescing, marked, with intratubular cocci, and mild hydronephrosis.
JPC Comment:
This week’s moderator was Dr. Enrico Radaelli from the University of Pennsylvania’s Comparative Pathology Core who led conference participants through a laboratory animal-centric conference with a few surprises. This first case is a succinct presentation with gross images and cytology that reinforce key slide interpretation. In particular, the history of urolithiasis and hydronephrosis is helpful in appreciating the irregular shape of the renal pelvis (especially in longitudinal section) and regional papillary necrosis which are discernable from the gross image. Gram-positive cocci are also numerous and consistent with the immunosuppressed state of this animal. We felt that pyelonephritis was the primary lesion in this case overall.
There was healthy debate among the group on our morphologic diagnosis. As the contributor notes, CPN is a common background lesion that inevitably encompasses changes at all levels of the nephron. We agree that these changes are present in this rat, though the severity was moderate for us compared with other conference cases we have reviewed (see Conference 23, Case 3, WSC 2012-2013 among others). We ran a Masson’s trichrome stain which highlighted the multifocal and irregular distribution of fibrosis which aligns with the limited degree of capsular/contour change in this case as well. The rat in this case was only nine months old at the time of euthanasia – for a comparison of CPN in a 2-year-old (24 months) rat and additional background literature, see Conference 12, Case 3, WSC 2019-2020.
Finally, Rag2 and IL-2Rγ warrant a brief discussion of their role in immunity. The common gamma chain (IL-2Rγ; γc) of the IL-2 receptor is a conserved and important component in intracellular signaling for multiple cytokines, including IL-2, IL-4, IL-7, IL-9, and IL-21.2,4 Once ligands are bound to these receptors, γc forms a heterodimer and recruits Janus kinase 3 (JAK3) to further propagate these signals via STAT and induce changes in gene expression. These cytokines are vital for mature lymphoid cell proliferation (IL-2), T-helper type 2 responses (IL-4), early lymphoid/hematopoietic progenitor cell development (IL-7, IL-9), and natural killer cell development (IL-15, IL-21).2,4 As such, knockout or mutation of IL-2Rγ (such as in X-linked SCID) results in significant impairment of NK, T-, and B-cells.6 Conversely, Rag2 forms one half (with RAG1) of a V(D)J recombinase that is essential for recombination of immunoglobulin and T-cell receptor variable regions that are needed for development of functional T- and B-lymphocytes.6 Defects in Rag2 are also associated with marked immunodeficiency. The combination of Rag2 and IL-2Rγ defects is a substantial and additive deficit of both innate and adaptive immunity.
References:
- Acuff NV, LaGatta M, Nagy T, Watford WT. Severe Dermatitis Associated with Spontaneous Staphylococcus xylosus Infection in Rag-/-Tpl2-/- Mice. Comp Med. 2017 Aug 1;67(4):344-349.
- Felsburg PJ, Hartnett BJ, Henthorn PS, Moore PF, Krakowka S, Ochs HD. Canine X-linked severe combined immunodeficiency. Vet Immunol Immunopathol. 1999 Aug 2;69(2-4):127-35.
- Frazier KS, Seely JC, Hard GC, Betton G, Burnett R, Nakatsuji S, et al. Proliferative and Nonproliferative Lesions of the Rat and Mouse Urinary System. Toxicologic Pathology. 2012;40-86S.
- Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993 Apr 9;73(1):147-57.
- Percy DH, Barthold SW, eds. Pathology of Laboratory Rodents and Rabbits. 4th ed. Blackwell Publishing; 2016:66.
- Perryman LE. Molecular pathology of severe combined immunodeficiency in mice, horses, and dogs. Vet Pathol. 2004 Mar;41(2):95-100.
- Salleng KJ, Jones CP, Boyd KL, Hicks DJ, Williams MM, Cook RS. Staphylococcus xylosus Cystitis and Struvite Urolithiasis in Nude Mice Implanted with Sustained-release Estrogen Pellets. Comp Med. 2018;68(4):256-60.