Signalment:
Gross Description:
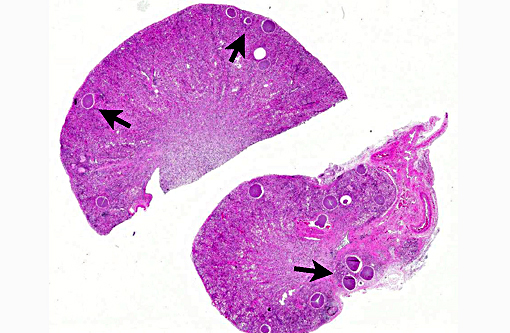
Histopathologic Description:
Morphologic Diagnosis:
Kidney: Protozoal cysts, numerous, etiology consistent with Besnoitia spp.
Kidney: Nephritis, interstitial, lymphoplasmacytic and neutrophilic, chronic, multifocal, marked, with membranous glomerulonephritis, and interstitial fibrosis.
Not submitted:
Haired skin, ovary, adrenal gland, spleen, and heart: Protozoal cysts, numerous, etiology consistent with Besnoitia spp.
Lung: Bronchopneumonia, granulomatous, multifocal, marked, with adult nematodes, larvae and eggs, etiology consistent with Capillaria spp. and Didelphostrongylus spp.
Spleen: Amyloidosis, multifocal, marked.
Condition:
Contributor Comment:
Besnoitia is a protozoal parasite in the phylum Apicomplexa.(3) Besnoitia spp. require two hosts (heteroxenous life cycle).(1,6) The domestic cat has been demonstrated to act as a definitive host for Besnoitia darlingi (species associated with opossums), in which infectious oocysts develop and are shed.(7) Opossums and other species act as intermediate hosts, in which oocysts develop into tissue cysts.(2,7) Opossums become infected by ingestion of oocysts from cats or tissue cysts by consumption of infected tissue from other intermediate hosts.(2,6,7)
JPC Diagnosis:
1. Kidney: Tubulo-interstitial nephritis, neutrophilic and histiocytic, chronic diffuse, marked with intratubular leptospires.
2. Kidney: Amyloidosis, interstitial, multifocal, mild.
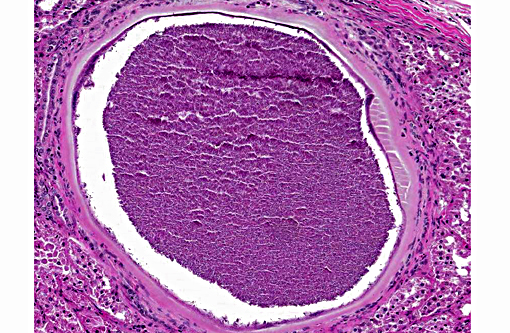
3. Kidney: Apicomplexan cysts, multiple.
Conference Comment:
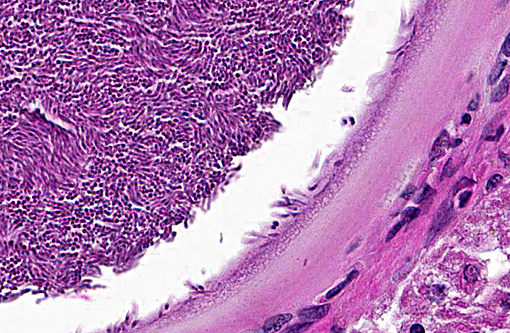
Histologically, the cysts consist of a single enlarged host cell, within which abundant crescent-shaped bradyzoites are packed into a parasitophorous vacuole, which fills the enlarged host cell. The host cell cytoplasm is present as a thin rim at the margin of the enlarged cell. Elongated host cell nuclei may be seen as an inner cyst membrane. The outer layer of the cyst, which forms the capsule, is seen as a variably thick, hyalinized layer of collagen fibers(2) which stains blue with Massons trichrome stain.(5) Mineralization of cysts may be seen as well as varying degrees of host inflammatory reaction.(2) Besnoitia spp. infections are well documented in other mammalian species, including cattle which may become infected with B. besnoiti. Recently, a new cyst nomenclature has been proposed for that species, due to historical inconsistencies in cyst descriptions consisting of the following: Hypertrophied host cell, enlarge nuclei, intracytoplasmic parasitophorous vacuole which contains bradyzoites, an inner cyst wall that may be vacuolated and an outer cyst wall in better developed cysts., and includes tissue cysts This entire structure is referred to as a tissue cyst. In cattle, the condition occurs in acute, subacute and chronic stages. The acute stage is associated with endothelial infections and resultant vascular damage. In subacute and chronic stages, tissue cysts are seen in mesenchymal host cells and are described in a variety of tissues, including being frequently described in the skin.(5)
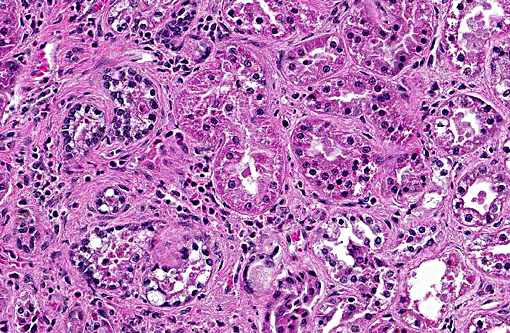
In this case, the primary lesions, aside from the cysts, include interstitial nephritis, fibrosis, and both tubular and glomerular damage. The interstitial infiltrate is diverse, ranging from predominantly lymphocytes and plasma cells in some areas to being neutrophil- and eosinophil-rich in others. There is mild multifocal intratubular inflammation with tubular degeneration and necrosis. Glomerular changes include membranoproliferative glomerulonephritis and glomeruli range from essentially normal to obsolescent; periglomerular fibrosis is striking in some areas. Amyloid is present in small amounts multifocally within the medullary interstitium, and during the conference, was confirmed with Congo red staining and green birefringence under polarization. The moderator also discussed the presence of extramedullary myelopoiesis, which is multifocal and extensive in some areas of the cortical interstitium.
In light of the interstitial nephritis and tubular changes, conference participants considered leptospirosis as a primary differential diagnosis. A Warthin-Starry stain identified low numbers of leptospires in the lumen of renal tubules. The opossum has been documented as a reservoir for multiple leptospirosis serovars. Renal lesions due to leptospirosis vary with virulence of the infecting serovar and stage of infection, but generally consist of varying degrees of tubulointerstitial nephritis and tubular necrosis. Subacute or chronic cases have an increased degree of interstitial inflammation and fibrosis may be extensive.(4)
References:
1. Ellis AE, Mackey E, Moore PA, Divers SJ, et al. Debilitation and mortality associated with besnoitiosis in four Virginia opossums (Didelphis virginiana). J Zoo Wildl Med. 2012; 43:367-374.
2. Elsheikha HM, Mansfield LS, Fitzgerald SD, Saeed MA. Prevalence and tissue distribution of Besnoitia darlingi cysts in the Virginia opossum (Didelphis virginiana) in Michigan. Vet Parasitol. 2003; 115:321-327.
3. Gardiner CH, Fayer R, Dubey JP, Service USAR. An Atlas of Protozoan Parasites in Animal Tissues. U.S. Department of Agriculture, Agricultural Research Service. 1988.
4. Greene CE, Sykes JE, Brown CA, Hartmann K. Leptospirosis. In: Greene CE. Ed. Infectious diseases of the dog and cat. 3rd ed. St. Louis, MO: Saunders Elsevier; 2006:402-415.
5. Langenmayer MC, Gollnick NS, Majzoub-Altweck M, Scharr JC. Naturally acquired bovine Bestoitiosis: Histological and immunohistochemical findings in acute, subacute and chronic disease. Vet Pathol. 2015; 52(3):476-488.
6. Shaw S, Grasperge B, Nevarez J, Reed S. Besnoitia darlingi infection in a Virginia opossum (Didelphis virginiana). J Zoo Wildl Med. 2009; 40: 220-223.
7. Smith DD, Frenkel JK. Besnoitia darlingi (Apicomplexa, Sarcocystidae, Toxo-plasmatinae): transmission between opossums and cats. J Protozool. 1984; 31:584-587.