Signalment:
Gross Description:
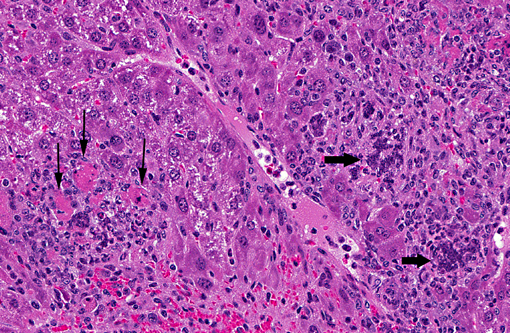
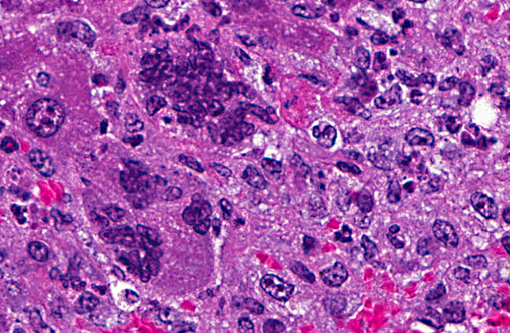
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
The pathogenesis of infection with a respiratory MHV strain begins with replication in the nasal epithelium, followed by dissemination via the blood stream to other tissues including liver, vascular endothelium, lymphoreticular tissues and brain, depending on host susceptibility. Pulmonary involvement is restricted to vascular endothelium and does not involve respiratory mucosa. The route of dissemination of infection to the brain is agedependent. In adult mice, infection tends to occur by extension of virus along the olfactory neural pathway. Microscopic findings are multifocal necrosis with syncytia in multiple organs, commonly including liver, spleen, lymph nodes and other lymphoid tissue(5). Hallmark syncytia of MHV infection are characterized by large degenerating cells at the periphery of necrotic foci, containing dense basophilic nuclei and nuclear remnants. Syncytia are more common and more well-developed in immunocompromised mice, as in this case.
Endemic infections are usually subclinical, sustained by continued arrival of naive susceptible animals (newborns), as there is generally no carrier state(1).
Immunocompromised mice infected with either respiratory or enterotropic strains of MHV may develop progressively fatal, multi-systemic infections with severe necrotizing lesions in nasal epithelium, vascular endothelium, brain, liver, bone marrow, lymphoid tissue and other sites(2). Virulent MHV strains kill these mice rapidly, but disease can be chronic with wasting in mice exposed to natural, avirulent strains of virus. Immunocompromised mice with only B cell defects infected with enterotropic MHV may develop enteric infections which are chronic but may not manifest overt clinical disease(2). High morbidity and mortality can develop with enterotropic MHV infection in neonatal mice in naive mouse colonies(5).
JPC Diagnosis:
1. Liver: Hepatitis, necrotizing, multifocal and random, with hepatic and endothelial viral syncitia, and capsular fibrosis.
2. Pancreas: Dochitis, necrotizing, diffuse, minimal.
Conference Comment:
Despite the name mouse hepatitis virus, polytropic MHV is not always hepatotropic. In addition to hepatic necrosis, common gross lesions include lymphoid tissue involution, ascites, hemorrhagic peritoneal exudate, necrotizing enterocolitis, thickened bowel segments in weanlings and adults, and mucosal proliferation or hyperplasia of the ascending colon and ileocecal junction in older mice. Microscopic lesions seen in other organs include intraepithelial eosinophilic intracytoplasmic viral inclusion bodies, necrotizing enterocolitis, segmental to diffuse villus blunting and atrophy at the ileocecal junction and ascending colon, and necrosis and syncytial giant cells of the splenic red pulp, lymphoid tissue, and hematopoietic tissues. In mice susceptible to neurotropic effects, there is necrotizing meningoencephalitis with spongiosis, demyelination, and syncytial giant cells in the central nervous system(5).
The differential diagnosis in mice for hepatitis and enteritis include:
a) Tyzzer's disease caused by Clostridium piliformis with intracytoplasmic bacilli and no giant cells
b) salmonellosis
c) mouse pox caused by ectromelia virus, an orthopoxvirus characterized by splenic necrosis (tiger striping) and skin lesions with intracytoplasmic eosinophilic viral inclusion bodies
d) epizootic diarrhea of infant mice (EDIM) caused by a rotavirus with less severe disease in neonatal mice with epithelial vacuolar degeneration in the ileum and jejunum, and epithelial eosinophilic intracytoplasmic viral inclusion bodies and no multinucleated giant cells or endothelial changes
e) reovirus-3, an orthoreovirus which causes foci of hepatic necrosis, CNS lesions, myocardial necrosis and pulmonary hemorrhage
f) adenovirus, which presents with intranuclear viral inclusion bodies
g) Helicobacter hepaticas, which causes proliferative colitis and rectal prolapse(3,5).
References:
2. Compton SR, Ball-Goodrich LJ, Johnson LK, Johnson EA, Paturzo FX, Macy JD: Pathogenesis of enterotropic mouse hepatitis virus in immunocompetent and immunodeficient mice. Comp Med 54:681-689, 2004.
3. Compton SR, Ball-Goodrich LJ, Zeiss CJ, Honson LK, Honson EA, Macy JD: Pathogenesis of mouse hepatitis virus infection in gamma interferon-deficient mice is modulated by co-infection with Helicobacter hepaticus. Comp Med 53:197-206, 2003
4. Maronpot RR: Pathology of the Mouse, pp. 96-97. Cache River Press, Vienna, IL, 1999
5. Percy DH, Barthold SW: Pathology of laboratory rodents and rabbits, 3rd edition, pp. 17-62, Blackwell Publishing, Oxford, UK, 2007.
6. Pritchett-Corning KR, Cosentino J, Clifford, CB: Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43: 165-173, 2009.
7. Pullium JK, Homberger FR, Benjamin KA, Dillehay DL, Huerkamp MJ: Confirmed persistent mouse hepatitis virus infection and transmission by mice with a targeted null mutation of tumor necrosis factor to sentinel mice, using short term exposure. Comp Med 53:439-443, 2003
8. Rehg JE, Blackman MA, Toth LA: Persistent transmission of mouse hepatitis virus by transgenic mice. Comp Med 51:369-374, 2001