WSC 22-23
Conference 20
Case II:
Signalment:
6 month-old, Hereford steer (Bos taurus)
History:
A 6-month-old Hereford steer presented to the Texas A&M Large Animal Food Animal Medicine and Surgery with a history of acute onset of being unable to rise. At presentation, the steer was recumbent and experiencing seizures. On neurological examination, he was ataxic, head-pressing and appeared blind. His condition was unresponsive to medical intervention, and the decision was made to humanely euthanize and perform a necropsy.
Gross Pathology:
Flared metaphyses of the ribs with retained calcified cartilage and mild cerebral edema.
Laboratory Results:
Blood lead level= 0.71 ppm (lab normal=0-0.3 ppm); Post-mortem analysis of renal tissue (after formalin fixation) = 6.2 ppm (>3 ppm indicative of toxicity). Radiographs of ribs revealed a flared physis and a radiodense band at the metaphysis.
Microscopic Description:
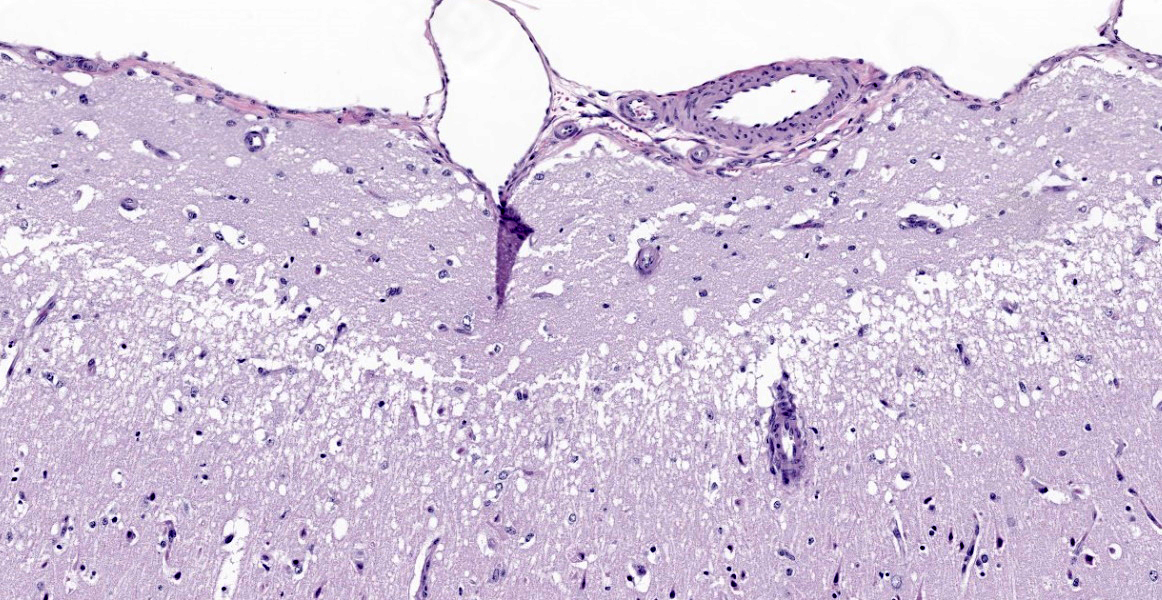
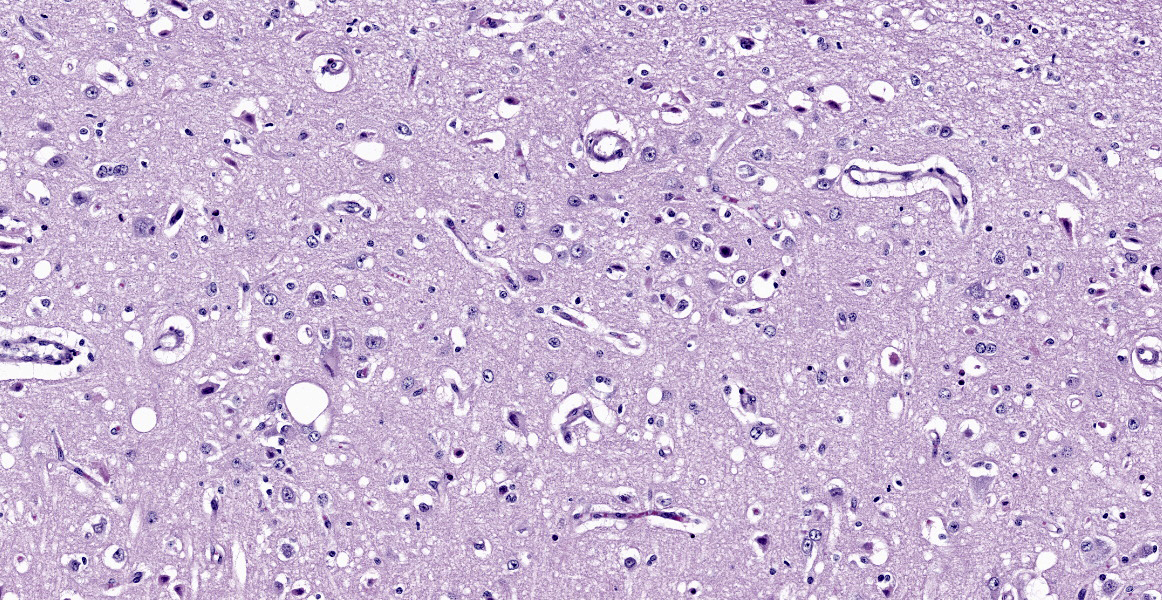
Cerebrum. The deep cerebrocortical interface has a laminar zone of rarefaction (edema), and many neurons are diffusely hypereosinophilic, shrunken/polygonal with pyknotic nuclei (neuronal necrosis) or are lost. Endothelial cells are swollen. Reactive astrocytes are numerous (gliosis). Occasional vessels have a few surrounding neutrophils. Rarely, the nuclei of neurons have 2-4 µm, round, eosinophilic (lead) inclusions.
Contributor’s Morphologic Diagnoses:
Severe, diffuse, laminar cortical neuronal necrosis with gliosis, edema and rare intraneuronal intranuclear eosinophilic inclusions (lead inclusions).
Contributor’s Comment:
The clinical signs related to lead poisoning are predominantly neurologic and include depression, inappetence, staggers, muscle tremors, bruxism, head pressing, convulsions, blindness, and death.1,14 Acute poisoning in cattle often results in death within 12-24 hours.1 Gross lesions are not striking in cases of lead poisoning but may include brain swelling, hemorrhage, or meningeal and cerebrovascular congestion.14 Microscopic lesions in acute cases may be confused with early autolysis.1 In subacute cases, microscopic lesions of laminar cortical necrosis of the cerebrocortical gyri, astrocytosis, perivascular edema or hemorrhage.14 Other differentials for bovine laminar cerebrocortical neuronal necrosis include, thiamine deficiency and consuming diets or water containing high sulphate (both causing ruminant polioencephalomalacia), or as a residual lesion of cyanide poisoning.1 This case is remarkable for the number of mitotic figures along with the significant neuronal necrosis. The mitotic population is presumably glial cells responding to the neuronal necrosis, but the population is negative with GFAP.
While multiple species may be affected with lead intoxication, cattle are 10 times more likely to be affected than dogs, cats, or horses.10 The disease is often acute in cattle and chronic in horses.1 Sheep are less commonly affected and swine are rarely affected.1 A retrospective, case-controlled study of cattle at North American veterinary school hospitals revealed a seasonality to lead intoxication cases with the most being reported in the late spring and summer months and an association between age and likelihood of exposure to lead with cattle less than four years of age at an increased risk.8 The highest risk age group are cattle between 2 and 6 months of age.8 Calves have higher exposure than adults, which is often from licking painted troughs, walls, etc. while confined. Adult cattle on pasture are often exposed through accidental ingestion of discarded car batteries, old lead paint on farm buildings or fences, or old asphalt shingles.4,14 Experimental models of lead intoxication do not reach the levels of naturally acquired lead intoxication, which may be due to the formation of lead salts that increases ingestion of lead contaminated objects.1 Other sources of environmental contamination include land near highways with historical lead accumulation in the soil from leaded gasoline exhaust, land near clay pigeon shooting ranges, industrial pollution, accumulation in plants, or fertilizers and pesticides.5,9,12
In lead intoxication the laminar necrosis does not autofluoresce under UV-light, suggesting a different pathogenesis from thiamine-related necrosis. The mechanism of action in lead intoxication is not well defined. The two theories of lead toxicosis are ionic mechanism and an oxidative stress mechanism. 2 The ionic mechanism proposes that lead metal ions cross the blood brain barrier using cationic transporters and replace other bivalent ions like Ca2+, Mg2+ or Fe2+.5,14 The direct neuronal toxicity is presumed to be due to altered function of the dopaminergic, cholinergic and glutamatergic neurotransmitter systems.14 Lead accumulates within neurons and astrocytes by utilizing a calcium transporter channel and disrupts calcium homeostasis, which leads to calcium release from the mitochondria with a subsequent increase in apoptosis. The oxidative stress mechanism proposes that lead toxicosis causes the levels of reactive oxygen species to increase while concurrently decreasing the antioxidant levels of proteins such as glutathione.5
Contributing Institution:
Dr. John Edwards and Dr. Martha Hensel (jedwards@cvm.tamu.edu; mhensel@cvm.tamu.edu)
Department of Veterinary Pathobiology
College of Veterinary Medicine and Biomedical Sciences
Texas A&M University
College Station, TX 77843-4467
JPC Diagnosis:
Cerebrum: Neuronal necrosis and loss, cortical, laminar, multifocal, with gliosis and edema.
JPC Comment:
This entity was last seen in Wednesday Slide Conference during Conference 10, Case 2 of 2021-2022. That case demonstrated lead toxicosis in the kidney of an African penguin, and readers are encouraged to review that case which details the features of disease in avian species and a brief review of the history of lead toxicity. During that conference, a recent Vet Pathol article was discussed which described lead intoxication in bald eagles; in this species, lead toxicosis leads to fibrinoid necrosis in arterioles of the heart, brain, and eyes leading to petechia, hemorrhagic necrosis, and ischemia in these organs.7
In the gross findings of this bovine case, the contributor describes flared metaphyses of the ribs and retained calcified cartilage, reflecting the effect of lead toxicity on bone. Lead inhibits the function of osteoclasts, which in growing animals normally resorb mineralized cartilage and woven bone of the primary spongiosa, making way for lamellar bone of the secondary spongiosa during endochondral ossification. 3 In lead toxicosis, osteoclasts fail to resorb bone and, histologically, osteoclasts are detached from the surface of trabeculae and may contain characteristic acid-fast eosinophilic occlusions.2 Grossly, there is a band of sclerosis and flaring of the metaphysis due to retained mineralized cartilage, and radiographically, this produces a characteristic lead line.2
Lead toxicosis can affect other systems as well. During erythropoiesis, lead inhibits heme synthesis and causes a nonregenerative, hypochromic, microcytic anemia with circulating sideroblasts. Lead also causes retention of RNA, leading to basophilic stippling.11 Lead quickly accumulates in the kidney and concentrates in the tubular epithelium nucleus, where inclusions are readily apparent (the moderator noted that eosinophilic intranuclear inclusions can be normal findings in healthy dogs). Lead inhibits mitochondrial respiration and in acute toxicosis can cause apoptosis of proximal tubular epithelium.6 In rabbits, lead toxicosis causes outer retinal degeneration secondary to lipofuscin buildup, and in rats, it causes necrosis of the photoreceptor cells and inner nuclear layer of the retina.13
Conference participants and the moderator debated on the presence of eosinophilic intranuclear inclusions and could not reach a consensus on whether they were present. Acid-fast staining by JPC did not confirm the presence in this case.
References:
- Cantile C, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals. 6th ed. Philadelphia, PA: Elsevier; 2016; 250-406.
- Craig LE, Dittmer KE, Thompson KG. Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. 6th St. Louis, MO: Elsevier. 2016; 23, 86.
- Gunson D, Groppe KE, Varela A. Bones and Jones. In: Wallig MA, Haschek WM, Rosseaux CG, Bolon Brad, eds. Fundamentals of Toxicologic Pathology.3rd 2018; 773.
- Hoff B, Boermans HJ, Baird JD. Retrospective study of toxic metal analyses requested at a veterinary diagnostic toxicology laboratory in Ontario (1990-1995). Can Vet J. 1998; 39(1):39-43.
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60-72.
- Khan KNM, Hard GC, Li X, Alden CL. Urinary System. In: Wallig MA, Haschek WM, Rosseaux CG, Bolon Brad, eds. Fundamentals of Toxicologic Pathology.3rd 2018; 250.
- Manning LK, Wünschmann A, Armién AG, et al. Lead Intoxication in Free-Ranging Bald Eagles (Haliaeetus leucocephalus). Vet Pathol. 2019;56(2):289-299.
- Mavangira V, Evans TJ, Villamil JA, Hahn AW, Chigerwe M, Tyler JW. Relationships between demographic variables and lead toxicosis in cattle evaluated at North American veterinary teaching hospitals. J Am Vet Med Assoc. 2008; 233(6):955-959.
- Payne JH, Holmes JP, Hogg RA, van der Burgt GM, Jewell NJ, Welchman Dde B. Lead intoxication incidents associated with shot from clay pigeon shooting. Vet Rec. 2013;173(22):
- Priester WA, Hayes, HM. Lead poisoning in cattle, horses, cats and dogs as reported by colleges of veterinary medicine in the United States and Canada from July, 1968, through June, 1972. Am J Vet Res.1974:35:567-569.
- Ramiah L, Bounous DI, Elmore SA. Hematopoietic System. In: Wallig MA, Haschek WM, Rosseaux CG, Bolon Brad, eds. Fundamentals of Toxicologic Pathology.3rd 2018; 329-334.
- Steuerwald AJ, Blaisdell FS, Geraghty CM, Parsons PJ. Regional distribution and accumulation of lead in caprine brain tissues following a long-term oral dosing regimen. J Toxicol Environ Health A. 201; 77(12):663-678.
- Teixeira L, Dubielzig RR. Special Senses - Eye. In: Wallig MA, Haschek WM, Rosseaux CG, Bolon Brad, eds. Fundamentals of Toxicologic Pathology.3rd 2018;725.
- Zachary JF. Nervous System Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier. 2012: 771-870.