CASE 3: 2019 Case 1 (JPC 4135948-00)
Signalment:
4.7 months old male Dunkin-Hartley guinea pig (Cavia porcellus)
History:
This guinea pig boar was obtained from the supplier with a jugular vein catheter and enrolled in an infectious disease imaging study. Following baseline imaging and bloodwork, this animal was experimentally infected followed by imaging and elective euthanasia on post-infection day 3.
Gross Pathology:
N/A
Laboratory results:
|
|
Baseline |
Terminal |
RI |
|
WBC |
6.25 |
4.47 |
3.12-8.71 |
|
Neutrophil |
2560 |
3490 |
932-3935 |
|
Lymphocyte |
3320 |
790 |
771-5058 |
|
Monocyte |
260 |
170 |
128-629 |
|
Platelets |
536 |
251 |
155-545 |
|
Hematocrit |
45.2 |
44.0 |
34.3-56.1 |
|
Glucose |
165 |
214 |
129-215 |
|
Creatinine |
0.4 |
0.7 |
0.2-0.7 |
|
BUN |
18 |
14 |
11-21 |
|
Calcium |
10.8 |
10.7 |
9.5-11.6 |
|
Albumin |
2.7 |
2.5 |
1.9-3.3 |
|
Total Protein |
4.7 |
4.7 |
4.0-5.8 |
|
ALP |
93 |
88 |
79-259 |
|
TBili |
0.3 |
0.3 |
0.2-0.4 |
|
GGT |
16 |
9 |
5-31 |
|
Amylase |
1093 |
1090 |
861-1613 |
Microscopic description:
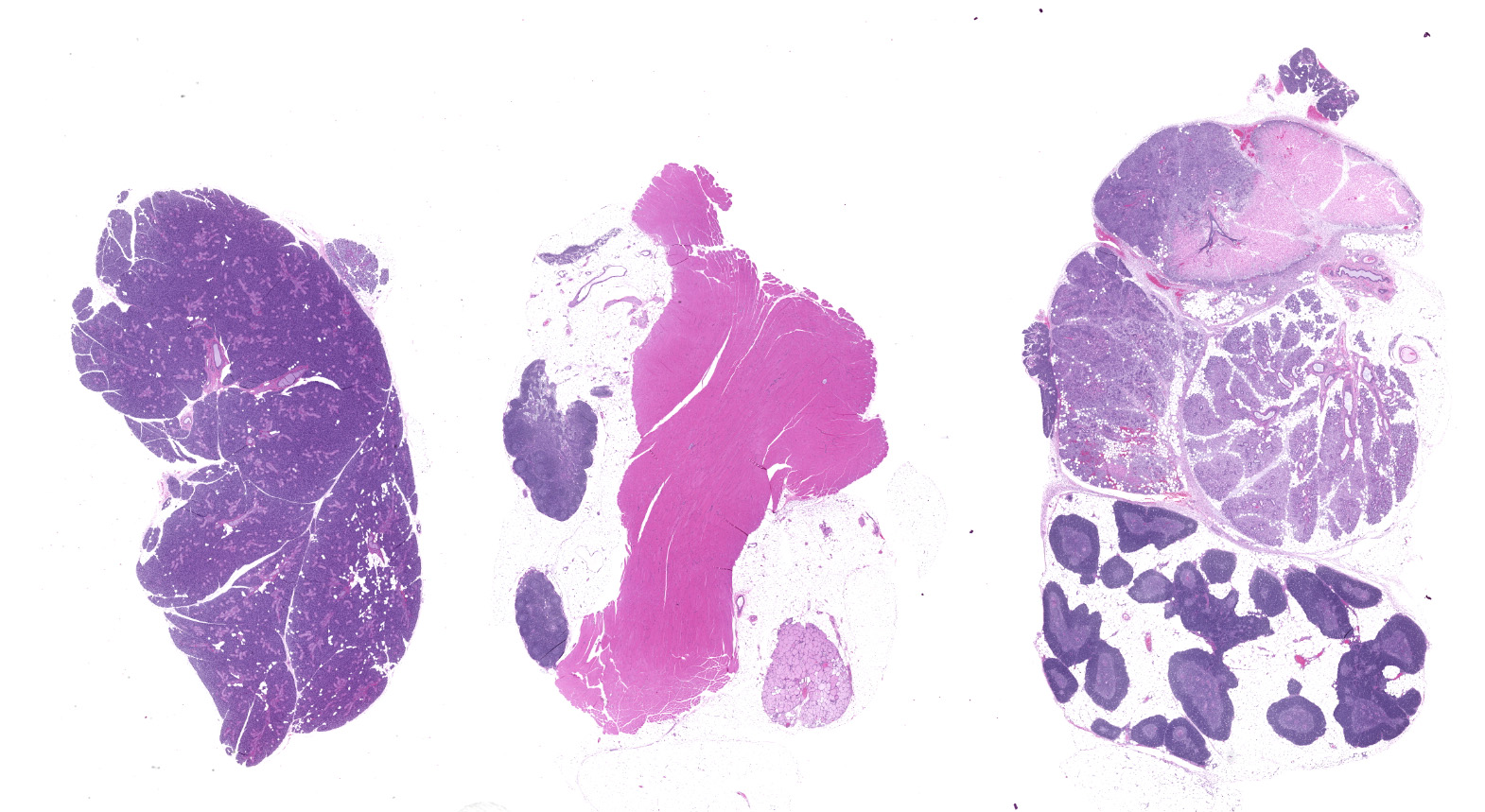
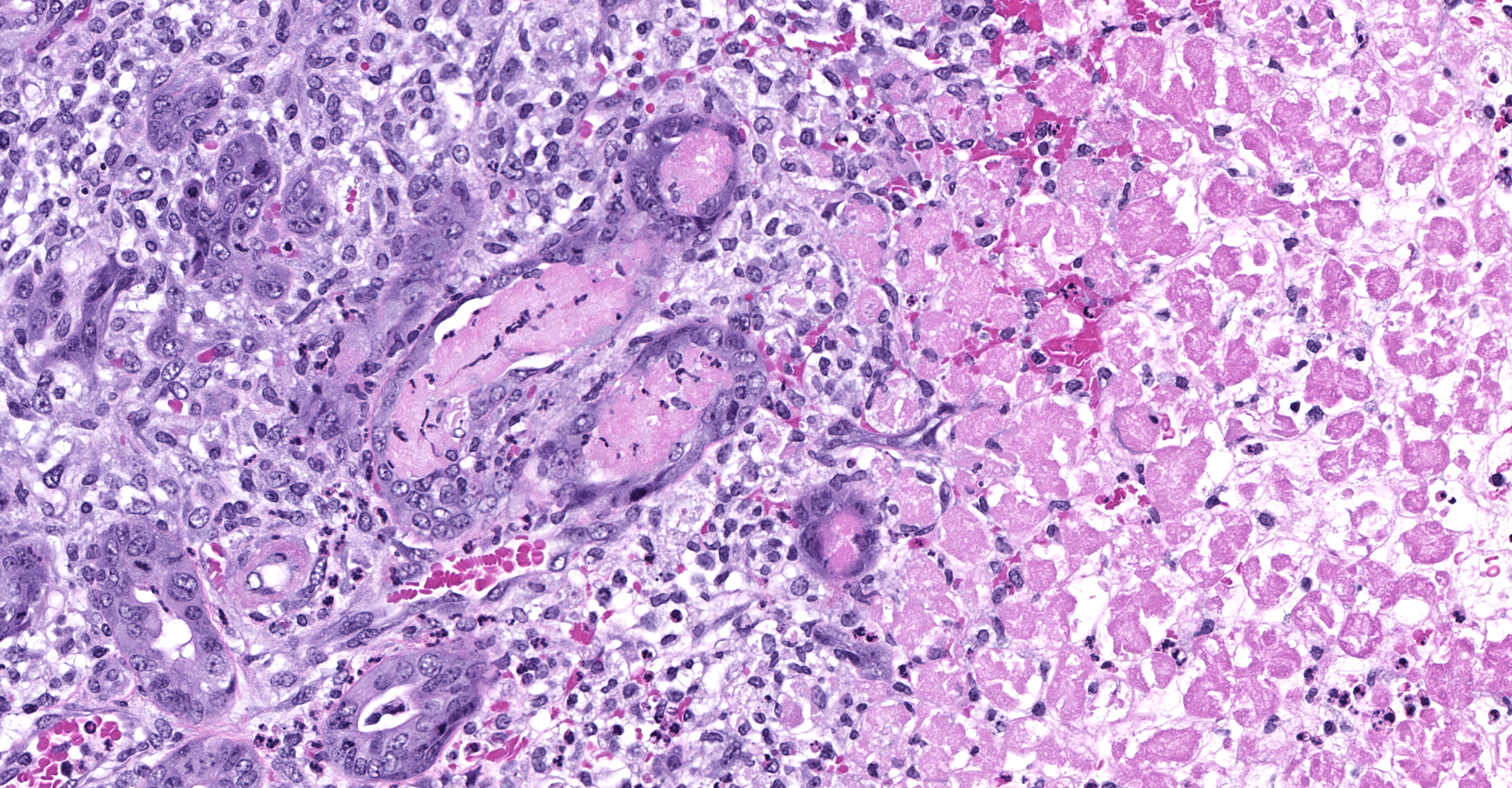
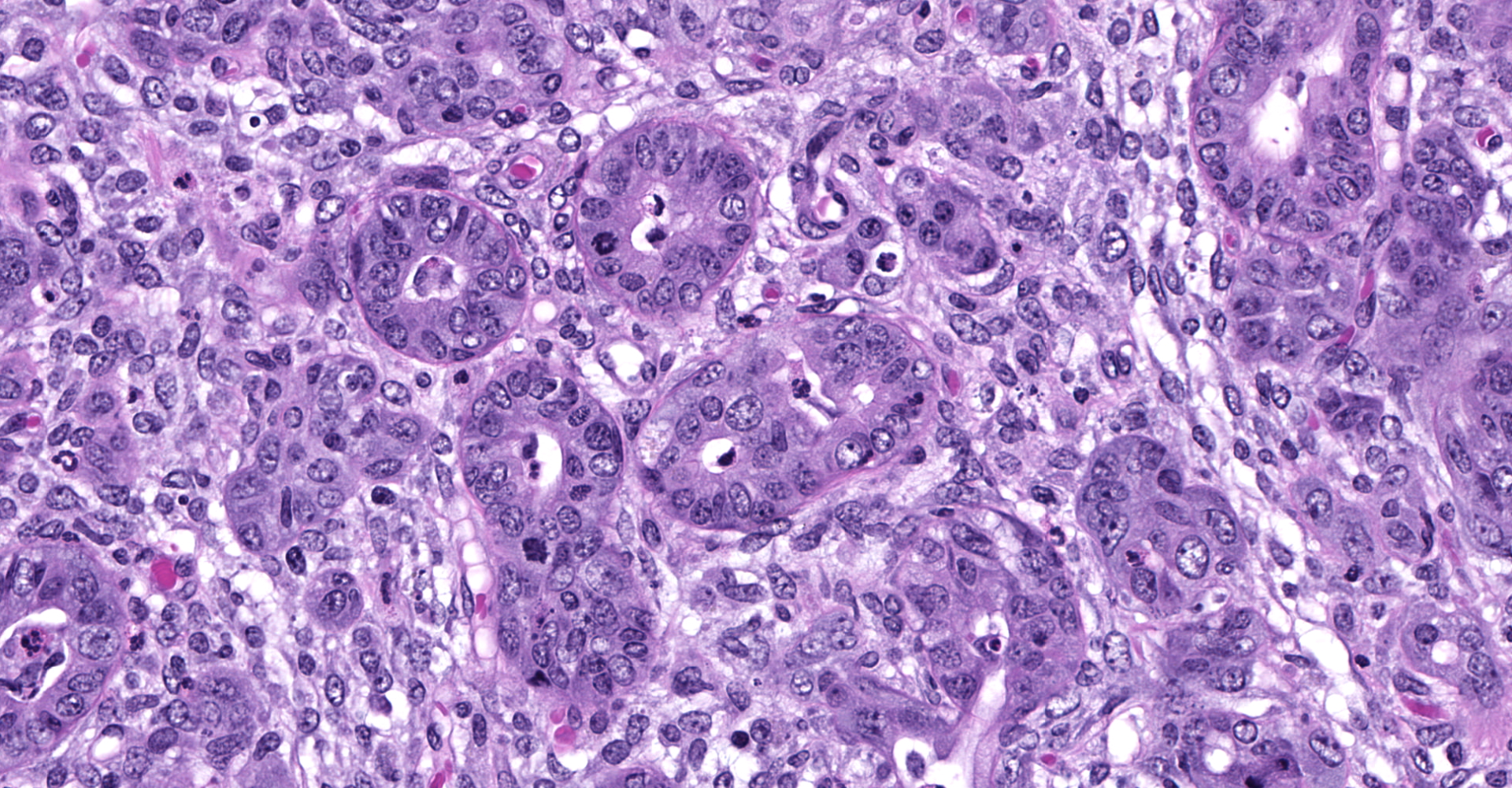
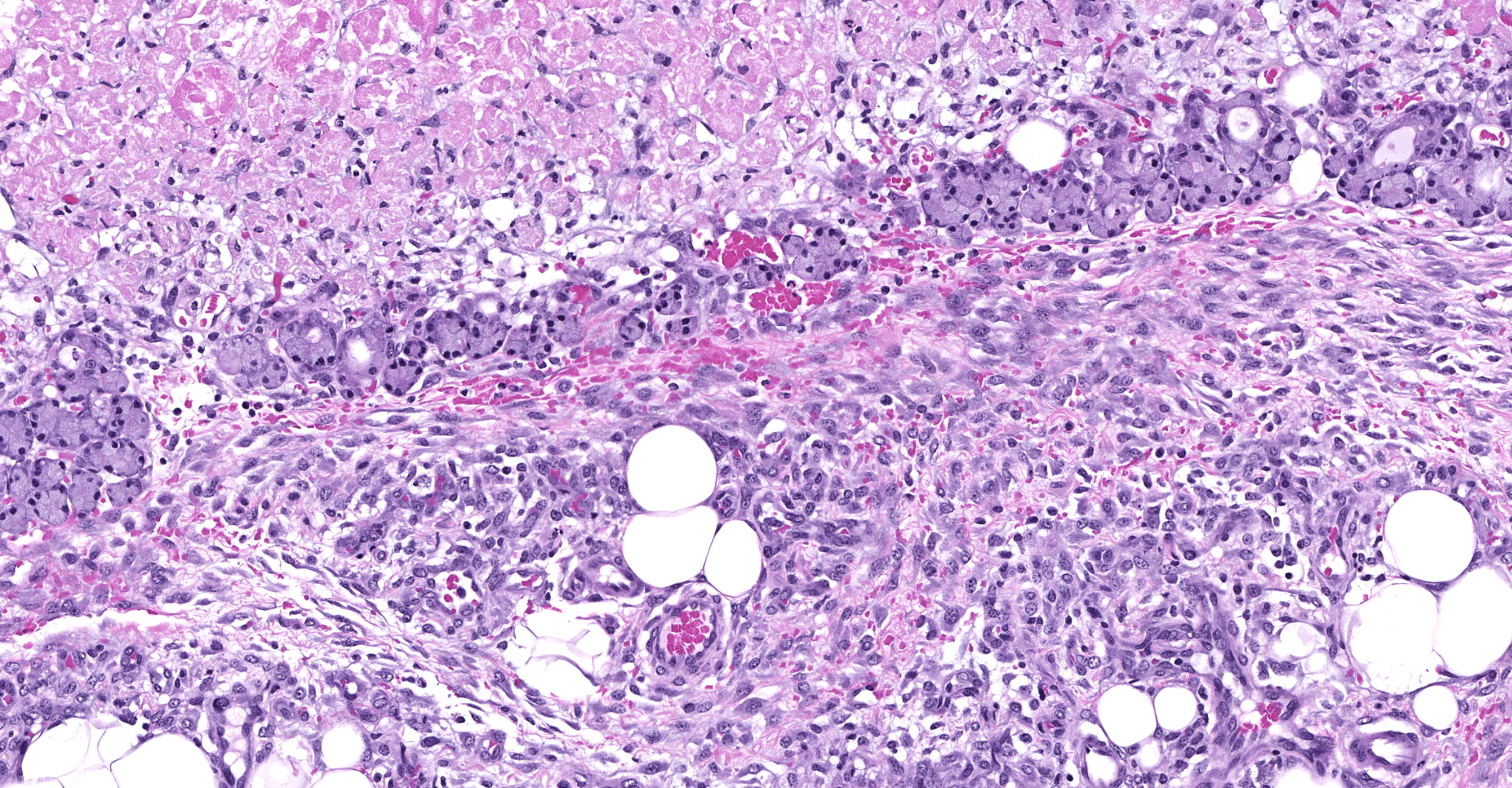
Slide contains sections of serous (parotid), mucinous (sublingual) and mixed (submandibular) salivary glands as well as thymus, cervical lymph nodes, thyroid, and skeletal muscle. Unilaterally affecting the parotid and sublingual glands there is focally extensive central coagulative necrosis and edema. At the margins of the lesions, with variable ingress to the center and replacement of infarcted tissues, there is florid infiltrate of densely cellular plump spindle cells (fibroblasts) with scant nascent edematous stroma, frequent macrophages and few neutrophils. Embedded within this process are numerous intralobular ducts, the morphology of which ranges from normal to crowded and stratified with cytoplasmic basophilia and frequent mitotic figures. There is scattered squamous metaplasia of the ducts with prominent desmosomes in the stratum spinosum. Some ducts within areas of coagulative necrosis are lined by attenuated basophilic cells with necrotic cell debris in the center. Acute fibrin thrombi or fragments of re-endothelialized and organized thrombus are visible in some small arteries, with plump hypertrophied endothelium lining the intima. Edema and nascent fibroplasia circumscribe both glands.
Contributor's morphologic diagnosis:
Parotid and sublingual salivary glands, unilateral, coagulative necrosis (infarct), focally extensive, subacute to chronic, severe with fibroplasia and ductal hyperplasia and squamous metaplasia
Contributor's comment:
Necrotizing sialometaplasia is a rare, poorly understood reactive lesion presumed to follow ischemic damage to the salivary gland. It has been previously reported in dogs, cats, and a laboratory rabbit.1,3,8,12 In humans, the lesion often affects the minor salivary glands of the palate, but can uncommonly affect major salivary glands.2,5 In addition to presenting as a mass lesion, dogs may have clinical signs including ptyalism, dysphagia and vomiting.4 It is important not to misdiagnose the process as squamous cell carcinoma with desmoplasia, particularly with cytology or small biopsy samples.
This guinea pig was obtained from the supplier at approximately 2 months of age with a catheter implanted in the right jugular vein. This procedure includes a percutaneous stay suture near the junction of the jugular vein and cranial vena cava. In this case, it is suspected that the suture may have inadvertently ligated the arterial supply to the ipsilateral parotid and sublingual salivary glands, resulting in infarction. The guinea pig salivary glands receive blood from the superficial cervical artery, maxillary artery, lingual artery, and cranial laryngeal artery.10 Based on location of the stay suture, it is likely that the superficial cervical artery was affected. The lesion was considered incidental and unrelated to experimental manipulations. It should be recalled that in guinea pigs the thymus is located in the cranioventral neck rather than the mediastinum.6
Contributing Institution:
Pathology Department
NIH/NIAID
Integrated Research Facility
https://www.niaid.nih.gov/about/integrated-research-facility
JPC diagnosis:
Salivary gland, submandibular: Coagulative necrosis (infarct), multifocal, with ductular degeneration, necrosis, and regeneration, squamous metaplasia, and acinar atrophy.
JPC comment:
In a recent analysis of 179 canine salivary lesions in Georgia (United States), approximately 2.2% of lesions were necrotizing sialometaplasia, with approximately half of cases diagnosed as non-specific sialoadenitis. Similar to this case, described cases of necrotizing sialometaplasia were characterized by extensive areas of coagulative necrosis bordered by mixed inflammation and fibrosis, vascular thrombosis, and duct hyperplasia with squamous metaplasia.7
Cases of necrotizing sialometaplasia have been reported in other species as well. Previous reports have indicated 5-13% of feline salivary lesions involve infarction, leading to necrotizing sialometaplasia. However, while human cases usually involve known causes of vascular damage or ischemia, a cause has not been identified in cats.1
In a study comparing the efficacy of different therapies for reducing the volume of the inferior nasal turbinate tissue in pigs, the 6 week post potassium titanyl phosphate (KTP) laser treatment patients had remarkable necrotizing sialometaplasia in the lamina propria, with cystic glands and excess mucous production. The KTP laser emits light at 532 nm, readily absorbed by hemoglobin, and causes endothelial impairment and thrombosis. The resulting coagulative necrosis is typically confined to a depth of 0.5 mm, with subsequent necrotizing sialometaplasia found in this region.11
The moderator and conference participants discussed the diagnostic criteria for this entity, which includes 1) massive infarction, 2) bland nuclear features, 3) squamous metaplasia of the ducts and acini, 4) inflammation and granulation tissue, and 5) maintenance of the glandular lobular structures. This entity may be misdiagnosed as mucoepidermoid, squamous cell carcinoma, or subacute necrotizing sialoadenitis.9,12
References:
1. Brown PJ, Bradshaw JM, Sozmen M, Campbell RH: Feline necrotising sialometaplasia: a report of two cases. J Feline Med Surg 2004:6(4):279-281.
2. Carlson DL: Necrotizing sialometaplasia: a practical approach to the diagnosis. Arch Pathol Lab Med 2009:133(5):692-698.
3. Kelly DF, Lucke VM, Denny HR, Lane JG: Histology of salivary gland infarction in the dog. Vet Pathol 1979:16(4):438-443.
4. Kelly DF, Lucke VM, Lane JG, Denny HR, Longstaff JA: Salivary gland necrosis in dogs. Vet Rec 1979:104(12):268.
5. Kim YH, Joo YH, Oh JH: A case of necrotizing sialometaplasia involving bilateral parotid glands. Am J Otolaryngol 2013:34(2):163-165.
6. Klapper CE: The development of the pharynx of the guinea pig with special emphasis on the morphogenesis of the thymus. Am J Anat 1946:78:139-180.
7. Lieske DE, Rissi DR. A retrospective study of salivary gland diseases in 179 dogs (2010-2018). J Vet Diag Invest. 2020;32(4):604-610.
8. Mukaratirwa S, Petterino C, Bradley A: Spontaneous necrotizing sialometaplasia of the submandibular salivary gland in a Beagle dog. J Toxicol Pathol 2015:28(3):177-180.
9. Shin SA, Na HY, Choe JY, et al. Necrotizing sialometaplasia: a malignant masquerade but questionable precancerous lesion, report of four cases. BMC Oral Health. 2020;20:206.
10. Shively MJ, Stump JE: The systemic arterial pattern of the guinea pig: the head, thorax, and thoracic limb. Am J Anat 1974:139(2):269-284.
11. Somogyari K, Moricz P, Gerlinger I, et al. Morphological and histological effects of radiofrequency and laser (KTP and Nd:YAG) treatment of the inferior turbinates in animals: A comparative pilot study. Surgical Innovation. 2016;24(1):5-14.
12. Villano JS, Cooper TK: Mandibular fracture and necrotizing sialometaplasia in a rabbit. Comp Med 2013:63(1):67-70.