Wednesday Slide Conference, 2025-2026, Conference 2, Case 1
Signalment:
28-year-old male castrated domestic pony (Equus ferus caballus)History:
A pony from a zoo in Michigan was diagnosed with choke on January 17th, 2020. On January 22nd, endoscopy was performed, an esophageal tear was suspected, and the animal was treated with Uniprim, procaine penicillin G, and phenylbutazone. The pony became febrile with a 103?F temperature and had multiple abscesses in the cervical area on ultrasound exam. The pony died with respiratory distress on January 28th when loaded on to the trailer for travel for repeat endoscopy.Gross Pathology:
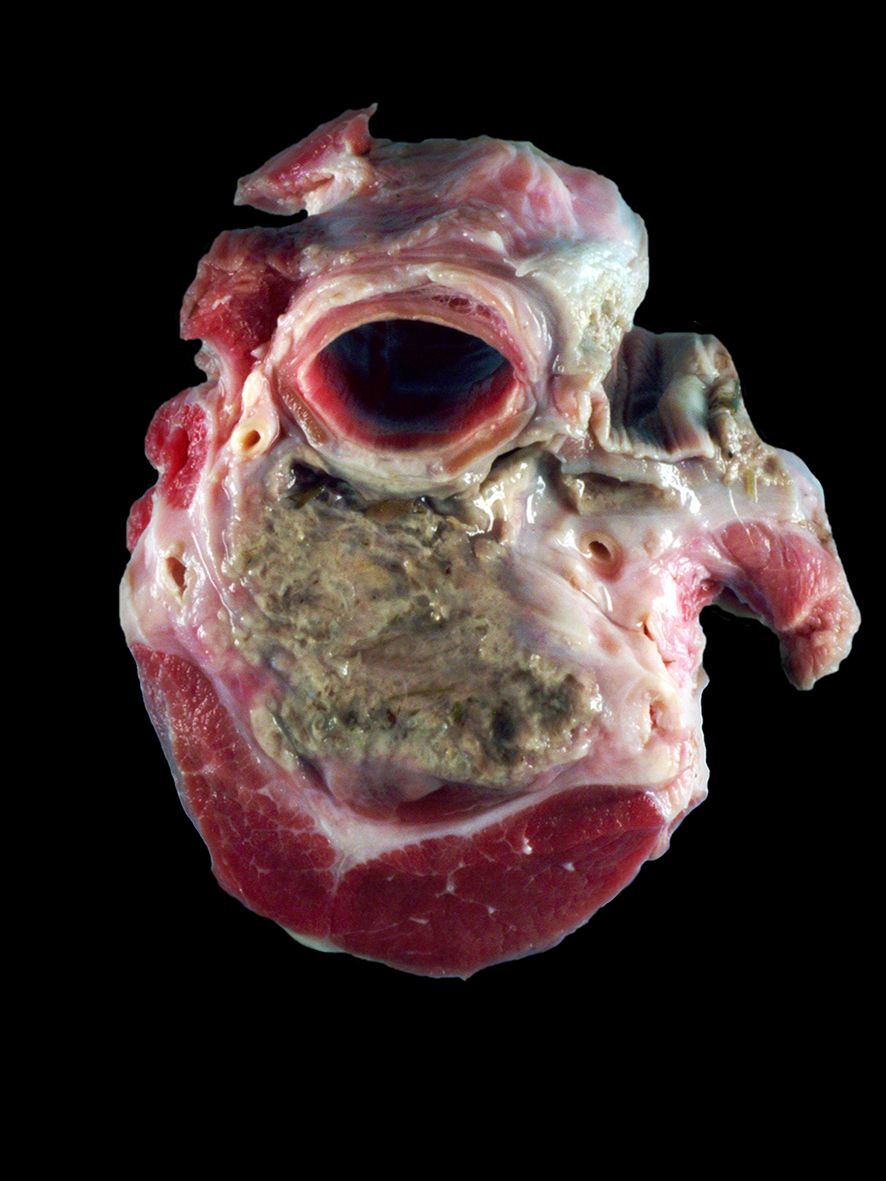
There was a 3 cm x 1.5 cm x 3.5 cm focal concretion of caseous material in the right serratus ventralis cervicis muscle. Fifteen to twenty multifocal similar lesions ranging from 6 cm x 0.75 cm x 0.5 cm to 2 mm x 2 mm x 1 mm were scattered throughout the cervical musculature. A 60 cm segment of serosa and tunica muscularis of the mid-esophagus was mottled black to purple. In the center of this affected area was a large cavitary space that extended approximately 10 cm into the surrounding cervical musculature and contained approximately 2 L of a suppurative exudate. The muscle surrounding the cavitary space was multifocally dark black and necrotic. There was no gross evidence of mucosal alterations in the esophagus, and no exudate was observed.Laboratory Results:
PCR for Acanthamoeba sp., Balamuthia mandrillaris, and Naegleria fowleri were negative. Aerobic culture yielded growth of moderate Actinobacillus spp, few Escherichia coli, and numerous Actinomyces spp. Anaerobic culture yielded numerous Bacteroides pyogenes, moderate Fusobacterium necrophorum, and numerous Porphyromonas sp. PCR for Streptococcus equi was negative.On cytology, there were large numbers of degenerate neutrophils. A heterogeneous population of extracellular bacterial rods and cocci, including some long filamentous rods, were either individualized or arranged in large aggregates. There were few round to oval organisms measuring approximately 1.5-2X the diameter of a neutrophil with a small purple eccentric nucleus and lightly to moderately basophilic cytoplasm that contained few clear vacuoles.
Microscopic Description:
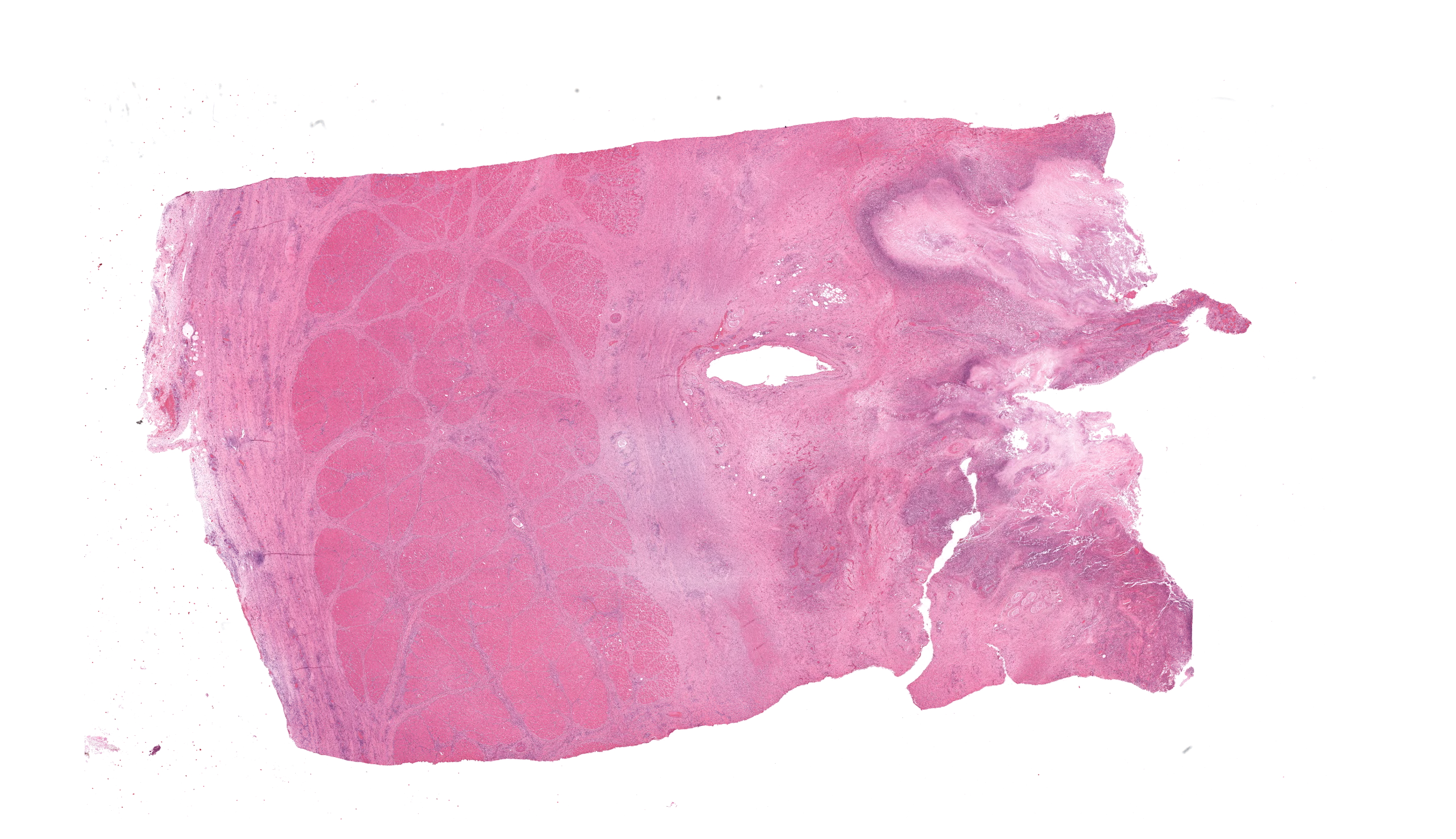
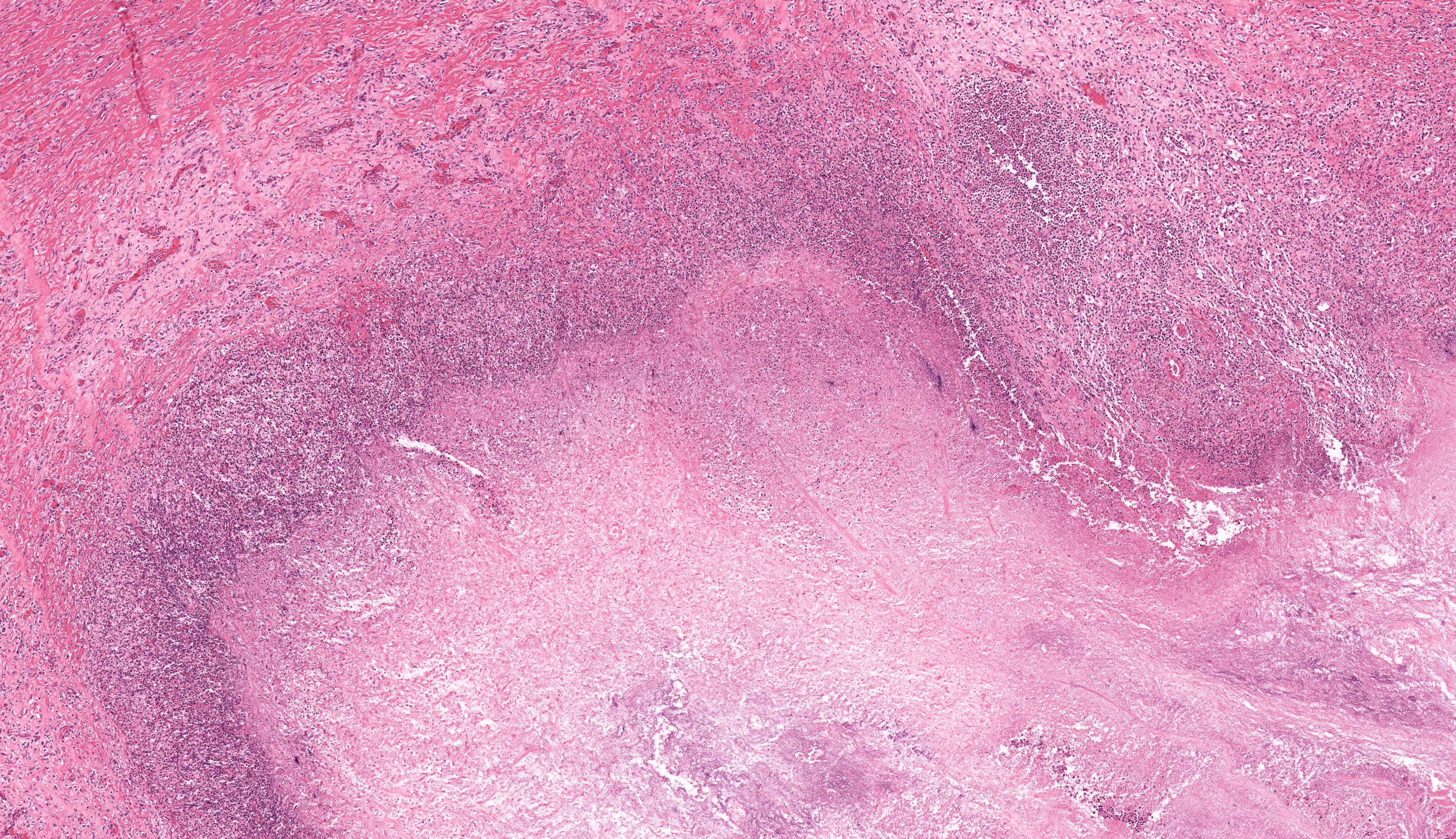
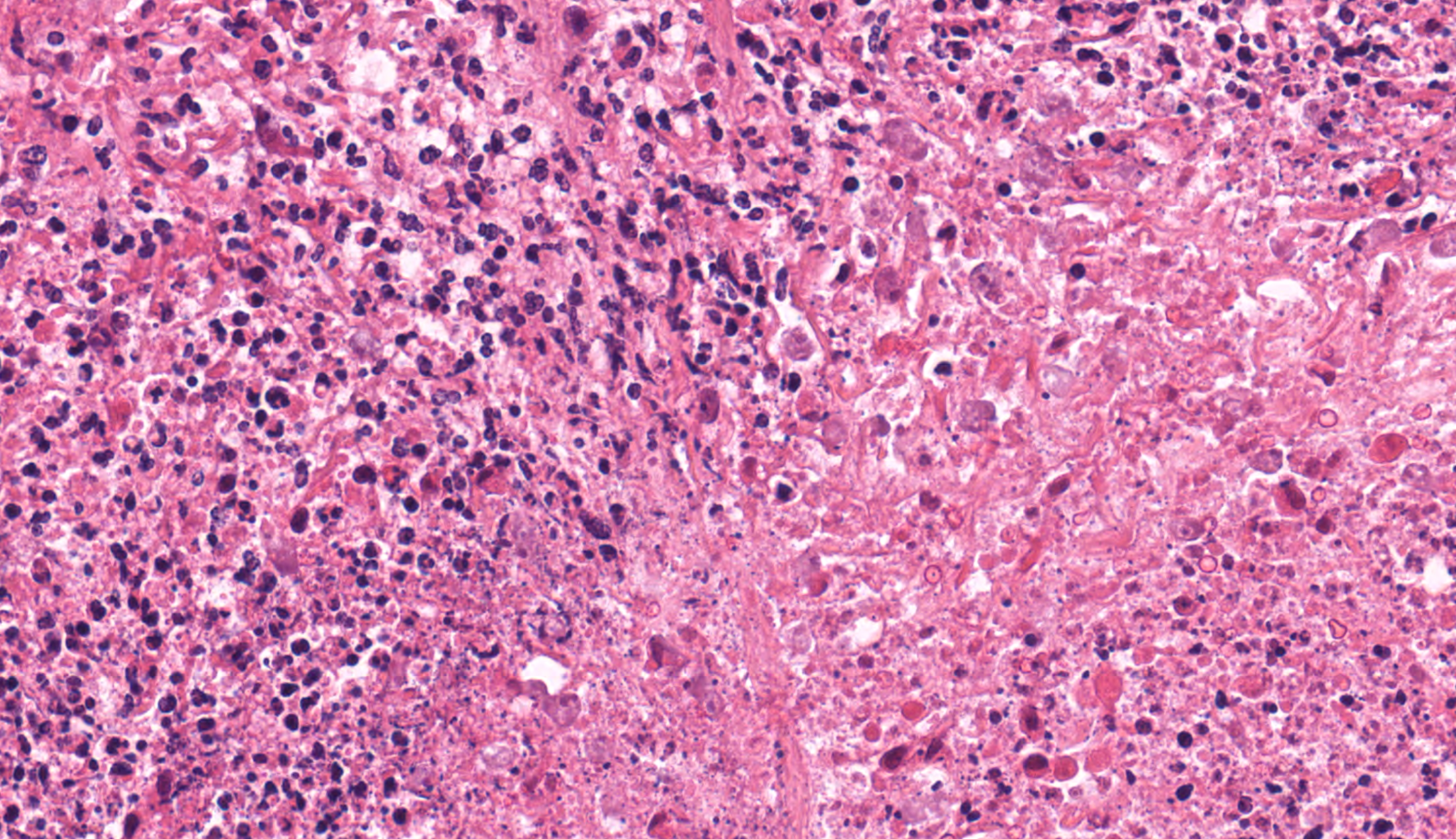
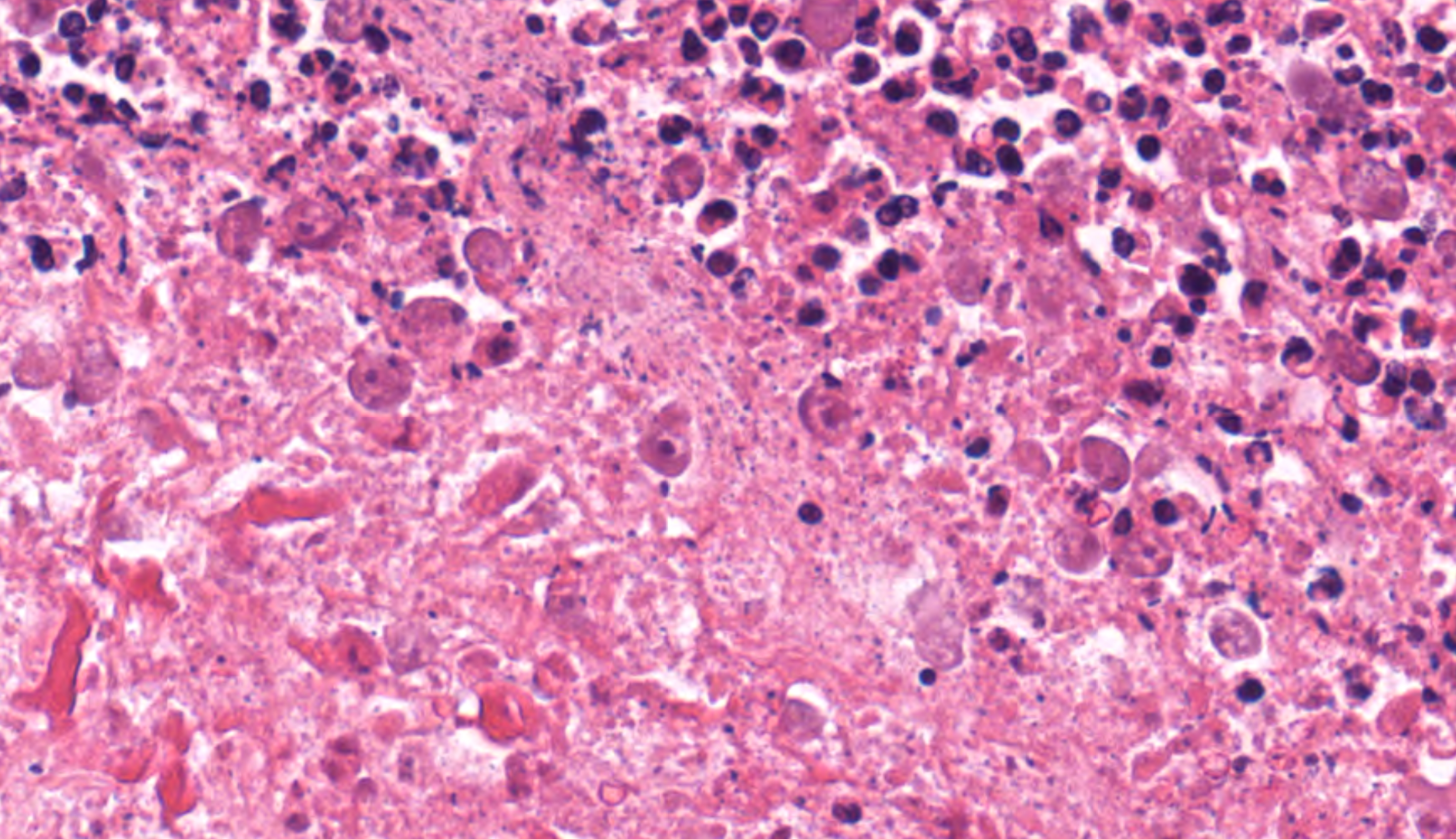
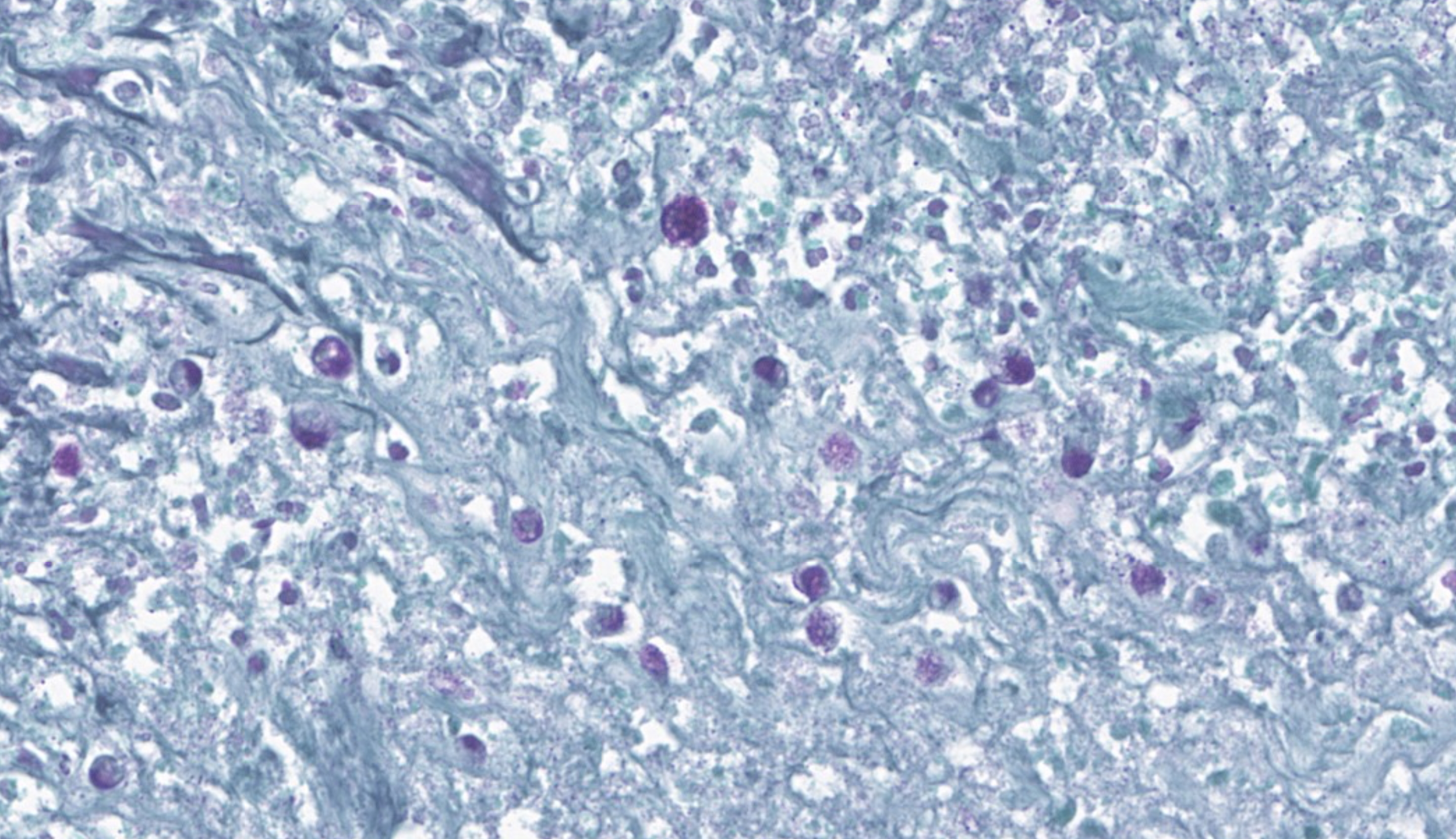
In multiple sections of cervical skeletal muscle, there were vast areas of liquefactive necrosis comprised of eosinophilic amorphous material, degenerated neutrophils and karyorrhectic debris. Within areas of necrosis were myriads of round amoebic trophozoites that were approximately 15um in diameter with a pale finely vacuolated to granular basophilic cytoplasm and eccentric eosinophilic 2-3um in diameter nucleus with a central karyosome and peripheralized chromatin. These organisms were variably degenerated. There were also numerous colonies of bacterial cocci and bacterial rods. Rimming these areas of necrosis was a band of degenerate neutrophils surrounded by a dense band of mature granulating fibrosis with fewer admixed lymphocytes, plasma cells, and histiocytes. Vessels in these areas were lined by plump reactive endothelium. Bands of fibrosis and lymphoplasmacytic inflammation extended into the skeletal muscle separating myofibers. Myocytes were multifocally shrunken and hypereosinophilic with loss of cross striation.Contributor's Morphologic Diagnoses:
Cervical muscle: Subacute, severe, locally extensive, necrotizing myositis with intralesional extracellular trophozoites, bacterial cocci, and bacterial rods.Contributor's Comment:
Amoebic myositis has rarely been reported in humans and animals and has only been associated with Entamoeba sp.2,4,12,16 In the two human cases, rupture of liver abscesses, perforation of intestines, or hematogenous or lymphatic spread of the organisms was the suspected route of infection.12,16 In these two cases, the amoebic myositis was caused by infections with Entamoeba histolytica, and in a single case in a monitor lizard, myositis was caused by Entamoeba invadens.2,12,16 In the monitor lizard, direct infection of a wound and hematogenous spread were determined to be the source of infection.2 Primary amoebic myositis has not previously been reported in horses and Entamoeba sp. has not been demonstrated as a primary pathogen in horses.3,6 Entamoeba sp. generally are a nonpathogenic commensal organism; however, they occasionally cause disease in animals that are immune suppressed, have concurrent disease, or experience a dramatic change in their intestinal flora.16 Entamoeba equi has been identified in the intestines of horses; but has not been associated with clinical disease.3,6 In animal species, the most commonly reported pathogenic entamoeba species are Entamoeba histolytica in primates and rarely dogs and cats, and Entamoeba invadens in reptiles.11,12,17In humans and primates, Entamoeba histolytica is considered a primary pathogen that causes gastrointestinal disease and is spread via the fecal-oral route.5 Clinical signs include bloody diarrhea, and the disease is responsible for 70 thousand human deaths a year.5 The trophozoites are located in the large intestine, and can spread through the intestinal wall leading to hematogenous spread.5 Entamoebic cysts are excreted in stool, and are ingested by the new host via contaminated food or water.5 Similarly, Entamoeba invadens is a major pathogen in reptilian species and can cause severe necrotizing hepatitis and enterocolitis in various snake species and chelonians.11,12 The pathophysiology of Entamoeba-associated diseases is linked to the important virulence factor in Entamoeba sp., the galactose and N-acetyl D-galactosamine (Gal-GalNAc) adherence lectin. This lectin allows the parasite to bind to the exposed Gal-GalNAc residues on the target cell glycoproteins.5Entamoeba sp. also form an amebapore, which is a channel-forming peptide.5 Once inserted into the target cell, this pore allows water and ions to enter the cell leading to lysis. Entamoeba sp. also produces cysteine proteases,5 which break down the extracellular matrix allowing for invasion of the organism.5
The suspected source of infection in the submitted case is a contaminated wound or injection site. The grossly observed white, inspissated material in multifocal areas of cervical muscle appeared microscopically as pools of pale eosinophilic crystalline material and most likely represent old injection sites. Clinically, an esophageal tear secondary to choke was suspected which may have represented a possible site of infection; however, no evidence of an acute or healing tear was observed grossly. Opportunistic infections of free-living amoeba have been reported in horses causing pneumonia, encephalitis, and placentitis.1,7,8 Considering the unusual location of the infection, a free-living amoeba was suspected as the cause of myositis; however, Acanthamoeba sp., Balamuthia mandrillaris, and Naegleria fowleri were excluded by PCR on formalin fixed paraffin embedded tissue.10 The water from the facility was also tested for free-living amoeba as there was concern for potential infections of other exotic species. No organisms were found in the tested water sample. Regardless, the histologic appearance of the trophozoites is most consistent with Entamoeba sp. as the nuclei have a central karyosome and peripheralized chromatin clumps.17 We were unable to confirm this by PCR as the available test was limited to fresh material due to the large size of the PCR target. Further characterization with additional primers or submission of fresh material for additional testing could not be performed due to the COVID-19 pandemic. A large component of the infection in this case was also bacterial. Organisms cultured in this case such as Fusobacterium sp., Bacteroides sp. and Actinomyces sp. are commonly associated with necrotizing myositis.4 Overall, the necrotizing myositis is suspected to be due to a combination of the effects of these bacteria and the Entamoeba sp.
Contributing Institution:
Michigan State University, Veterinary Diagnostic Laboratory, 4125 Beaumont Road, Lansing MI 48910JPC Diagnoses:
Skeletal muscle: Rhabdomyositis, necrotizing, chronic-active, focally extensive, severe, with numerous amoebic trophozoites.JPC Comment:
Kicking off the second conference in this year's lineup is the Joint Pathology Center's very own Dr. Bruce Williams! (Please hold your applause until the end.) Supplemented by an excellent contributor write-up and an AIgenerated image of a psychedelicantamoeba(courtesy of Dr. Williams; please don't hesitate to contact him for this image as it is truly... something), this case made for a tough diagnostic challenge for conference participants. Who would have thought Entamoeba sp. in a pony's neck!?
Conference discussion focused on the five ?famous amoebae? that pathologists should have mentally filed away for a rainy day, which are Entamoeba histolytica, Entamoeba invadens, Balamuthia mandrillaris, Naegleria fowleri, and Acanthamoeba spp. In addition, participants were put through their paces to name the primary species affected, organs affected, and diseases caused by these five. The main morphologic differences between a protozoal trophozoite and a protozoal cyst were covered and can be distilled down to trophozoites having more indistinct cell borders, a single nucleus, and exhibiting phagocytosis whereas cysts are round with distinct borders and 4+ nuclei. A rather unfortunate reference was made to the fact that, in humans, amoebic abscesses used to be called ?anchovy paste abscesses?, which is something that absolutely no one needed to be informed of.... ever. The last point of discussion was generated by a question from a conference participant asking why a karyosome is called such when it so closely resembles a nucleolus? To answer that inquiry, a nucleolus is a membrane-bound organelle in the nucleus of a cell responsible for making ribosomal RNA (rRNA) and producing ribosomes. A karyosome, by contrast, is a clustered bunch of chromatin and protein found within the nucleus and is often associated with the nucleolus. Hypothetically, they should sometimes both be able to be seen histologically within amoebic trophozoites.
There are scattered reports of amoebic infection in horses primarily involving ?free-living? amoeba species, in particular Acanthamoeba and Balamuthia spp.8 However, Entamoeba histolytica and E. moshkovskii have also been reported to cause severe disease, and these have both been isolated in rare cases from horses. E. histolytica, the most widely studied member of its species; that most notably affects humans and non-human primates, is equipped with a variety of nasty virulence factors that enable it to cause devastating disease in its host. As a generality, most Entamoeba sp. are non-pathogenic, lacking the same virulence factors as pathogenic Entamoeba sp. Even pathogenic Entamoeba sp. don’t generally cause issues unless there is a drastic change in the host’s gastrointestinal microflora, which are the primary nutrient source for Entamoebasp. within the host. If that happens, all bets are off. Think of these pathogenic Entamoeba sp. as tiny, living bombs with anger management problems that are kept docile only by giving them their favorite foods to eat…if they no longer can feed on those preferred bacteria, they exist the GI lumen by invading the intestinal walls and, in a blind rage, start blowing up everything in their paths. From there, they can go wherever the vascular system takes them.
The virulence factors that allow them to do so include the Gal/GalNAc lectin, which is a protein that allows the Entamoeba to adhere to host cells, including erythrocytes and GI epithelial cells, and the protective mucus layer of the GI tract.17 The Gal/GalNAc lectin is the primary determinant in Entamoeba’s ability to invade tissues9. Additionally, it has poreforming peptides known as “amoebapores” that the Entamoeba shoves into 7host cell membranes, resulting in cell lysis.9,17 Amoebapores are basically the offensive, singleprotein, protozoal version of our own multiprotein, defensive complement cascade. This amoebapore enables the Entamoeba to kill host cells and invade tissues.9 Once within the tissue, E. histolytica secretes a variety of cysteine protease (CPs) enzymes that degrade host tissue components, including intestinal mucus and extracellular matrix.9 Specific CPs have been linked to pathogenicity, such as CP5 in humans, which enables the induction of apoptosis in host target cells. In the face of all this destruction, the host immune system utilizes, among other things, oxidative stress to try and fight off the invaders. However, Entamoeba histolytica has an uncanny ability to resist oxidative stress by using a combination of antioxidant enzymes, such as peroxiredoxin and superoxide dismutase, and by making metabolic shifts towards glycerol and chitin biosynthesis, both of which are additionally protective against free O2 radicals13. Finally, as if this protozoon needed any additional advantages, Entamoeba histolytica engages in “trogocytosis”, in which the trophozoite ingests small “bites” of living host cells. It then processes and displays host cell membrane proteins on its own surface, which function like a cloaking device and gives the Entamoeba a +10 stealth boost against host immune cells. With all these virulence factors primed for pure devastation, it should come as no surprise that the National Institute of Allergy and Infectious Diseases (NIAID) classifies E. histo lytica as a category B priority biodefense pathogen. The contributor made note that they also cultured three different bacteria from this case: Fusobacterium necrophorum, Actinomyces sp., and Bacillus fragilis. Each of these bacteria is considered an opportunistic commensal, and conference participants largely regarded them and their potential effects as secondary to the damage wrought by the Entamoeba. However, they are worth briefly discussing. necrophorum, once inoculated into an anaerobic environment (such as an area of necrosis), can cause devastating lesions via multiple virulence factors, including a leukotoxin, endotoxin, haemolysin, haemagglutinin, and adhesin. Among these, leukotoxin and endotoxin are believed to be the more important toxins in overcoming the host's defenses15. F. necrophorum is frequently seen in mixed infections and synergisms between it and other pathogens are considered important in causing disease. Bacillus fragilis, now known as Bacteroides fragilis, which usually resides in the intestines, is a common cause of peritonitis and sepsis from intestinal integrity issues or diseases, including post-operative complications.19 It, too, has multiple virulence factors at its disposal, such as fragilysin (also known as BFT, or Bacteroides fragilis toxin), capsular polysaccharides that promote abscess formation, make the bacteria more resistant to degradation, and increasing bacterial aggregation, as well as several enzymes, including hyaluronidase, chondroitin sulfatase, deoxyribonuclease, proteases, phosphatases, and lipases, which allow attack and penetration of the host’s extracellular matrix.19 The production of hemolysins is thought to facilitate access of B. fragilis to iron and heme in vivo via damaged host cells and erythrocytes. Lastly, and showing up empty-handed to the community potluck of devastation, is Actinomyces sp. Actinomycosis infections are usually polymicrobial and they are generally considered opportunistic bystanders, with few notable exceptions, who don’t really contribute much but get to reap the benefits of just being there. All in all, it was the opinion of conference participants that Entamoeba was the star of this show, followed around by three enthusiastic bacterial groupies to cause the worst case of not-actually-choke choke ever.
References:
- Begg AP, Todhunter K, Donahue SL, Krockenberger M, Slapeta J. Severe amoebic placentitis in a horse caused by an Acanthamoeba hatchetti isolate identified using next-generation sequencing. J Clin Microbiol. 2014:52(8):3103-3104.
- Chia MY, Jeng CR, Hsiao SH, Lee AH, Chen CY, Pang VF. Entamoeba invadens myositis in a common water monitor lizard (Varanus salvator). Vet Pathol. 2009; 46:6733-676.
- Clark CG, Kaffashian F, Tawari B, et al. New insights into the phylogeny of Entamoeba species provided by analysis of four new small-subunit rRNA genes. International Journal of Systemic and Evolutionary Microbiology. 2006;56:22352239.
- Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3)473-494.
- Espinosa-Cantellano M and Martinez-Palomo A. Pathogenesis of intestinal amebiasis: From molecules to Disease. Clin Microbiol Rev. 2000; 13(2)318-331.
- Jacob AS, Busby EJ, Levy AD, Komm N, Clark CG. Expanding the Entamoeba universe: New hosts yield novel ribosomal lineages. J Eukaryot Microbiol.2016;63:69-78.
- Kinde H, Read DH, Daft BM, et al. Infections caused by pathogenic free-living amebas (Balamuthia mandrillaris and Acanthamoeba sp.) in horses. J Vet Diagn Invest. 2007;19:317-322.
- Kinde H, Visvesvara GS, Barr BC, Nordhausen RW, Chiu PWH. Amebic meningoencephalitis caused by Balamuthia mandrillaris (leptomyxoid ameba) in a horse. J Vet Diagn Invest. 1998;10:378381.
- Nakada-Tsukui K, Nozaki T. Immune Response of Amebiasis and Immune Evasion by Entamoeba histolytica. Front Immunol. 2016 May 12;7:175.
- Norgan AP, Sloan LM, Pritt BS. Detection of Naegleria fowleri, Acanthomoeba spp, and Balamuthia mandrillaris in formalinfixed, paraffin-embedded tissues by realtime multiplex polymeras chain reacton. Am J Clin Pathol. 2019;152:799-807.
- Ossiboff RJ. Serpentes. In: Terio KA, McAloose D, St. Leger J, ed. Pathology of Wildlife and Zoo Animals. 1st ed. London, UK: Academic Press.
- O’Leary C and Finch R. Amoebic psoas and liver abscesses. Postgrad Med J. 1992;68:972-973.
- Rastew E, Vicente JB, Singh U. Oxidative stress resistance genes contribute to the pathogenic potential of the anaerobic protozoan parasite, Entamoeba histolytica. Int J Parasitol. 2012;42(11):1007-15.
- Rodriguez CE, Duque AMH, Steinberg J,Woodburn DB. Chelonia. In: Terio KA, McAloose D, St. Leger J, ed. Pathology of Wildlife and Zoo Animals. 1st ed. London, UK: Academic Press.
- Tan ZL, Nagaraja TG, Chengappa MM. Fusobacterium necrophorum infections: virulence factors, pathogenic mechanism and control measures. Vet Res Commun. 1996;20(2):113-40.
- Tetiker T, Sert M, Tuncer I, et al. An unusual amebic localization on the right hip. Infection. 1995; 23:124-125.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 6th ed. Vol 2. St. Louis, MO: Elsevier
- Yanagawa Y, Sharma M, Izumiyama S, Singh U. Exploring virulence and stress response in Entamoeba histolytica: insights from clinical strains. Microbiol Spectr. 2025;13(7):e0050625
- Yekani M, Baghi HB, Naghili B, Vahed SZ, Sóki J, Memar MY. To resist and persist: Important factors in the pathogenesis of Bacteroides fragilis. Microb Pathog. 2020;149:104506.