Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 24
10 April, 2019
CASE I: W837-12 (JPC 4088206)
Signalment1 day old, female, Quarterhorse, equine (Equus caballus)
History: The foal was presented for development of colic several hours after birth. The foal was white with small areas of grey/black coloration on the tail base, lip and ear; the mare had a solid bay coat with a small area of white flecking on the left shoulder and the stallion was paint. The foal was suckling but passage of meconium was not observed. Reduced gastrointestinal sounds were noted on auscultation, but the examination was otherwise unremarkable. No meconium was palpated within the rectum. A moderate amount of intestinal gas was noted on abdominal radiographs. The colic failed to resolve with supportive treatment and the abdomen became progressive distended and tympanic over the next 12 hours. Due to continued deterioration, the foal was euthanized and submitted for post-mortem examination.
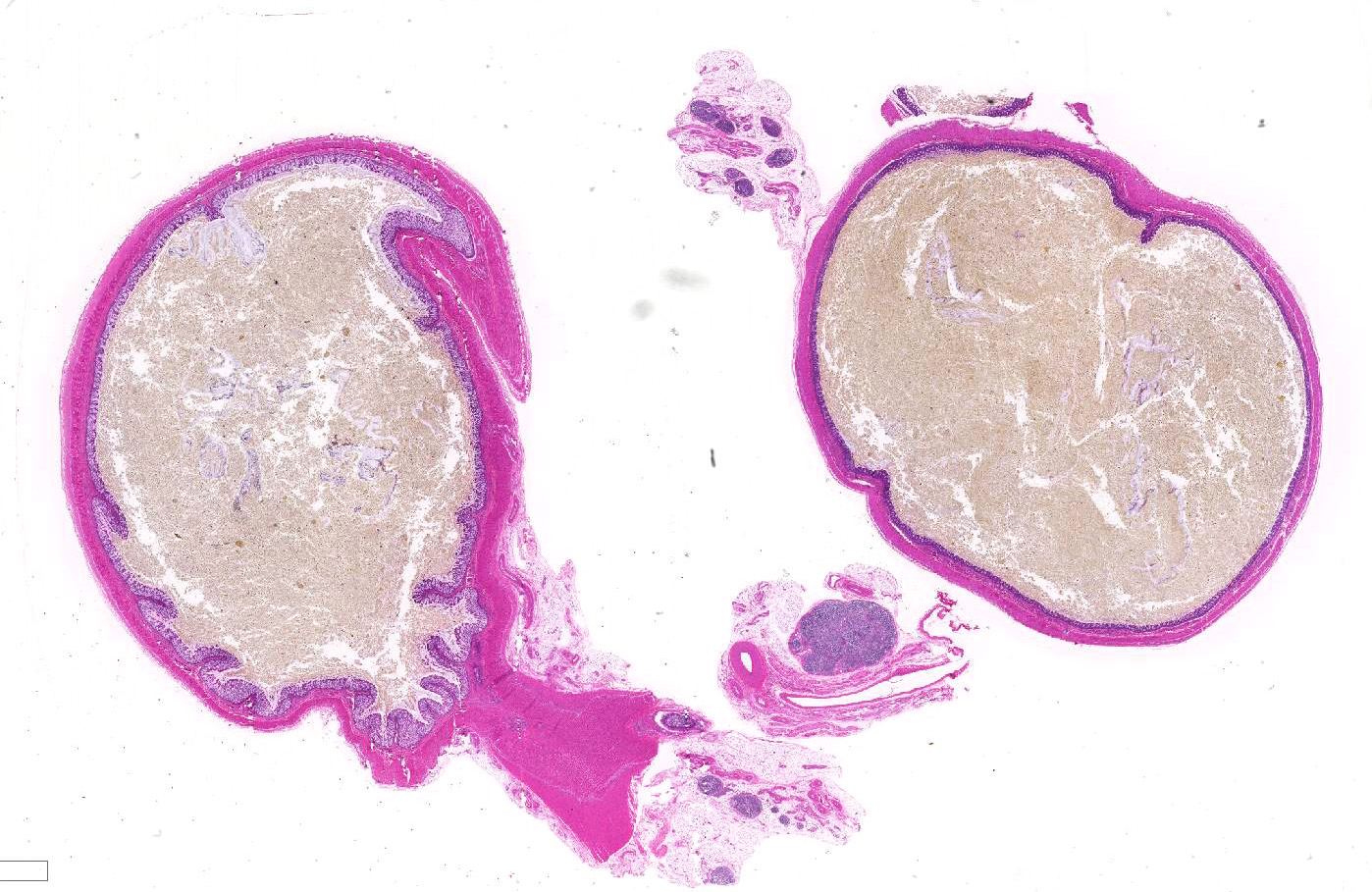
Gross Pathology: The rectum and small colon appeared to be diffusely small and pale. The large colon was filled with meconium and also appeared to be segmentally mildly stenotic along the right dorsal section, though the entire gastrointestinal tract was patent. The small intestine was distended with gas and liquid meconium distally, while the stomach and proximal duodenum contained ingested milk. All other body systems were unremarkable.
Laboratory results: NA
Microscopic Description:
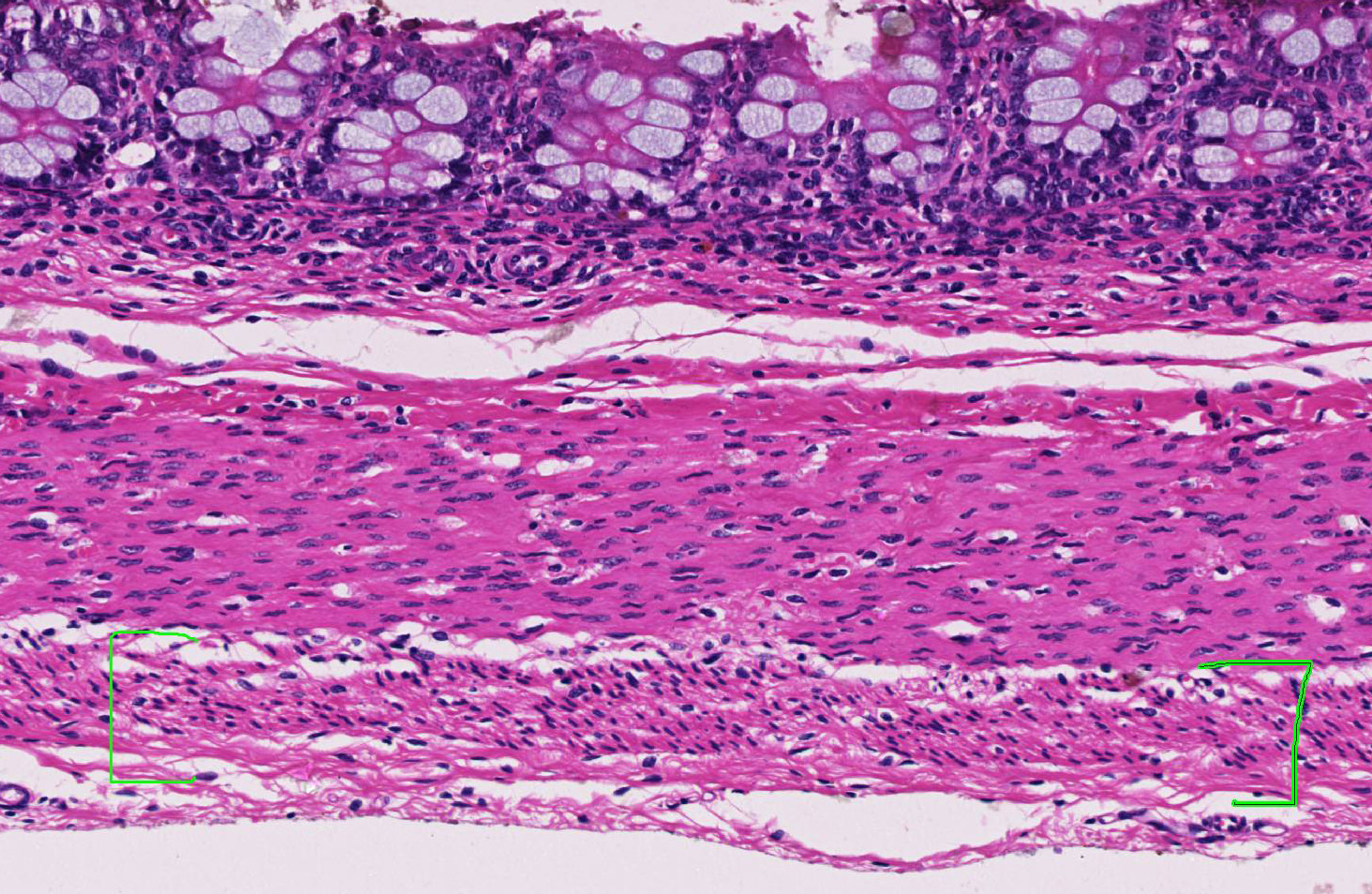
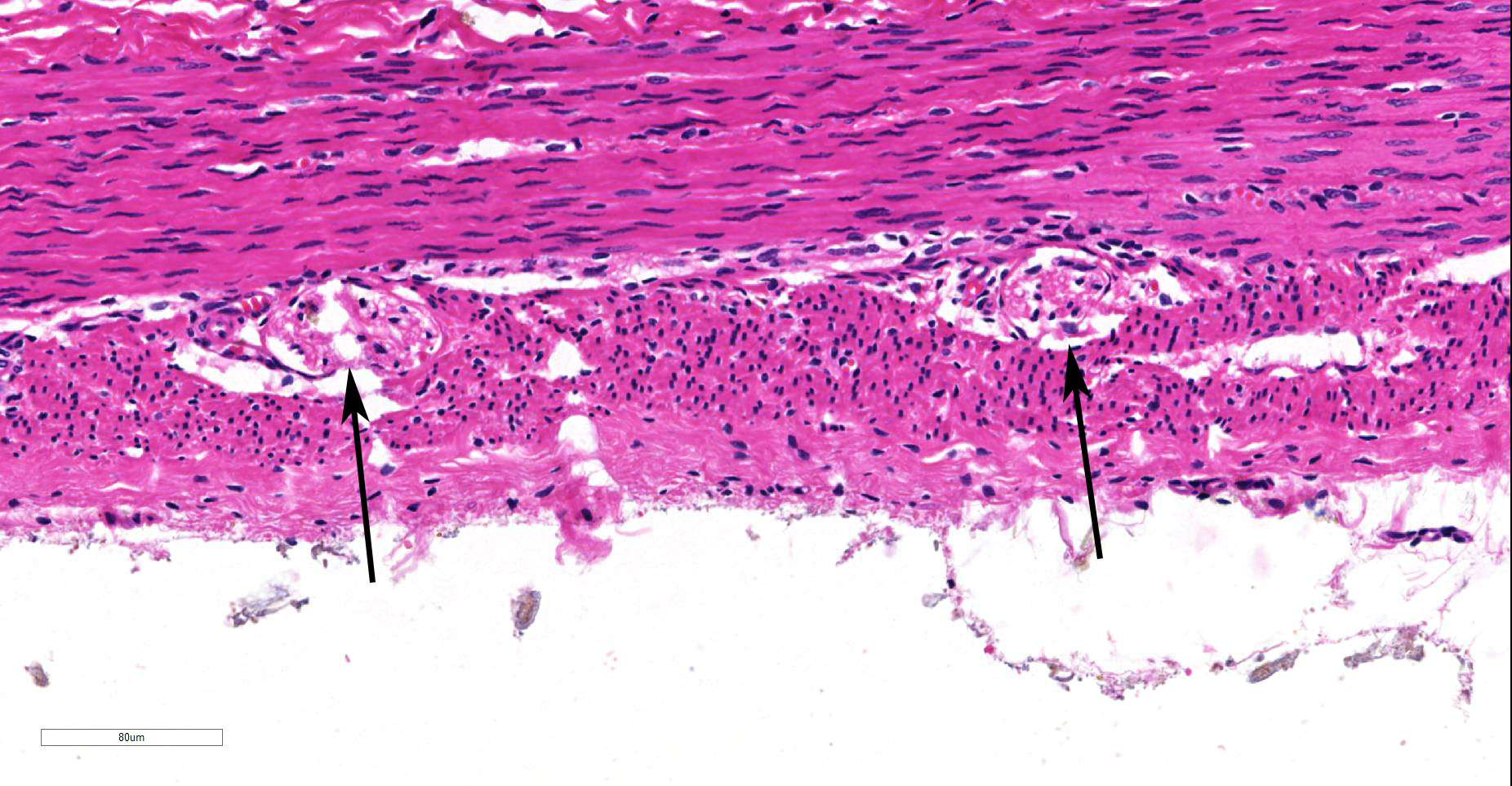
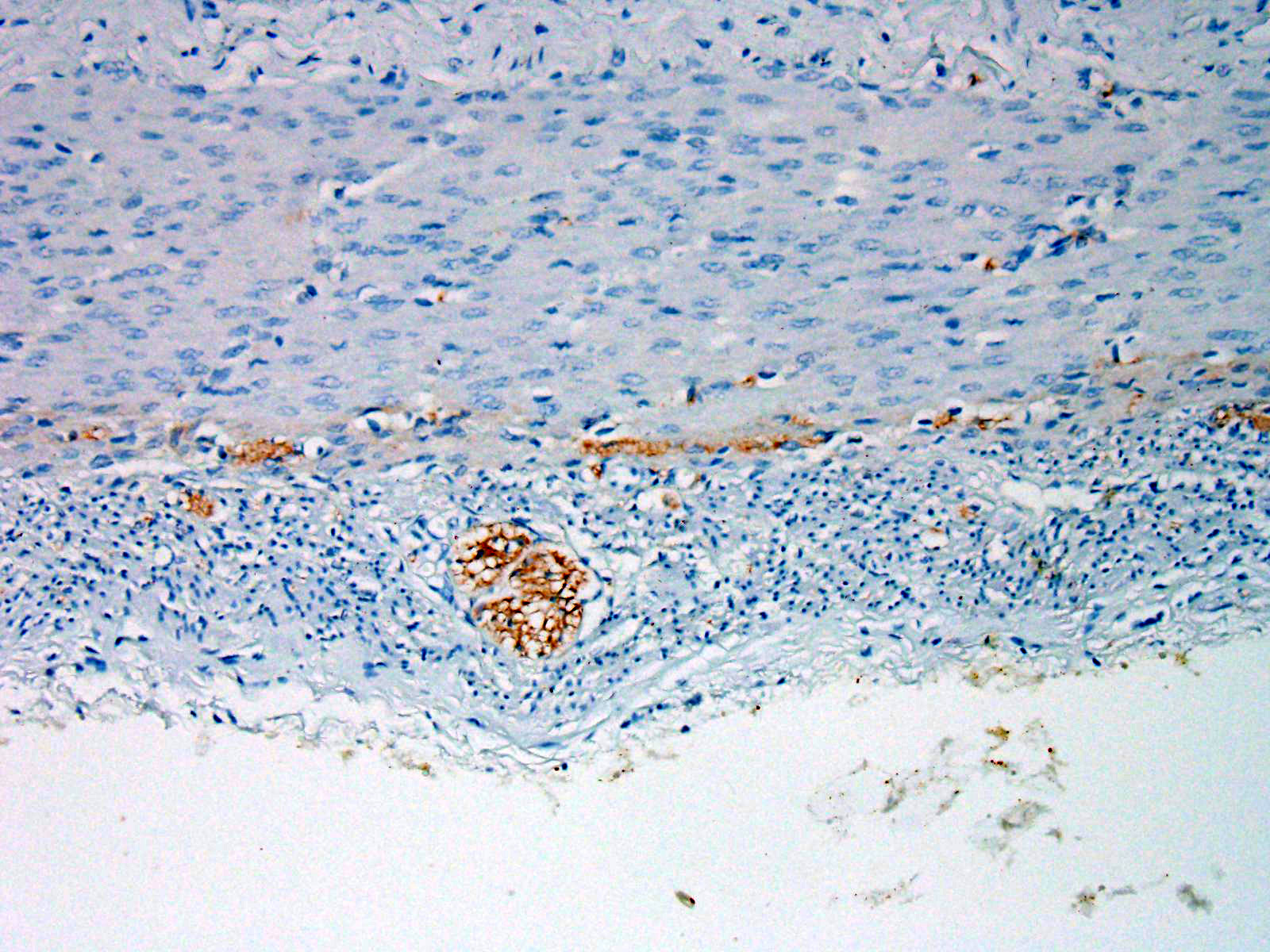
Within the colon, there is complete absence of ganglion cells within the gut wall, together with a severe paucity of myenteric (Auerbach’s) ganglia and submucosal nerves (Meissner’s). The submucosa was reduced, but in the tissue that remained there was increased prominence of stromal cells.
Contributor’s Morphologic Diagnoses:
Ileocolonic aganglionosis, severe, diffuse, chronic (consistent with overo lethal foal syndrome)
Contributor’s Comment: Lethal White Foal Syndrome (LWFS) is a disease associated with horse breeds that register white coat spotting patterns. Breeding between particular spotted horses, generally described as ‘frame overo’, produce some foals that, in contrast to their parents, are all white or nearly all white and die shortly after birth of severe intestinal blockage.1 The frame overo coat pattern is described as white markings on the lateral and ventral aspects of the neck and torso, whereas a pattern with more white on the dorsal cervical and lumbar regions and the legs is called ‘tobiano’.3.
Overo lethal white syndrome occurs in newborn foals is associated with the ‘overo’ coat pattern that receives a copy of the mutated OLW gene (Ile118Lys EDNR-B) from each parent.8 Two heterozygote carriers of the mutated gene must be mated to produce a homozygous lethal white foal. Color patterns with highest incidence (> 94%) of heterozygotes were frame overo, highly white calico overo, and frame blend overo. White-patterned bloodlines with lowest incidence of heterozygotes (< 21 %) were tobiano, sabino, minimally white calico overo, splashed white overo, non-frame blend overo, and breeding-stock solid. The mutation was not detected in solid-colored horses from breeds without white patterning.4,6,8
Phenotypically, the altered gene causes lack of skin pigmentation and white coat colour which was strongly associated with endothelin receptor B (EDNR-B) genotype. Endothelin receptors are important in neural crest cell migration. Neural crest cells are precursor cells to a variety of cell types including melanocytes, neural and glial cells of the peripheral, including the enteric, nervous systems.9 A consequence of a foal carrying two copies of the mutated EDNR-B gene is that neural crest cells do not migrate to the skin or the gut appropriately, thus resulting in foals being born with a white hair coat and aganglionosis of the submucosal and myenteric ganglia of the distal part of the small intestine and of the large intestine, resulting in intestinal immotility and colic, similar to human Hirschsprung’s disease.3,4
The condition Hirschsprung's disease in human children, seen in 1 in 5000 live births, and can be familial or sporadic. In these children often only a segment of the colon or the distal rectum is involved and leads to congenital megacolon. Familial Hirschsprung's disease has been associated with one or more mutations in three separate genes: 1) the RET proto-oncogene, 2) the endothelin-3 (EDN-3) gene, and 3) the endothelin-B receptor (EDNR-B) gene.2 Endothelin-3 knockout mice have congenital megacolon due to colonic aganglionosis and coat color spotting due to regional lack of epidermal melanocytes. Endothelin-B receptor knockout mice have the same phenotype.4 Mutation of this receptor gene has also been shown to be responsible for familial Hirschsprung's disease in Mennonite families.6 Affected humans also occasionally exhibit abnormalities in skin pigmentation.
White patterning in horses has been strongly associated with EDNR-B genotype. Determination of EDNR-B genotype by use of a DNA-based test is the only way to determine with certainty whether white-patterned horses can produce a foal affected with OLWS.3 (3).
DNA-based test that identifies horses that are heterozygous for the Overo lethal white gene has been developed. The allele-specific polymerase chain reaction test locates and amplifies the specific mutated site in the endothelin receptor B gene (EDNR-B gene).3 The test for this mutant allele can be utilized in all breeds where heterozygous animals may be unknowingly bred to each other including the Paint Horse, Pinto horse, Quarter Horse, Miniature Horse, and Thoroughbred.
Contributing Institution:
Veterinary Pathology, Faculty of Veterinary and Agricultural Sciences, University of Melbourne http://www.fvas.unimelb.edu.au
JPC Diagnosis: Colon: Aganglionosis, diffuse, severe, with marked hypoplasia of the muscular tunics.
JPC Comment: The contributor has done an excellent job in describing the molecular basis of lethal white foal syndrome and Hirschsprung’s disease, its human equivalent. Hirschsprung’s disease was named after Danish pediatrician Harald Hirschsprung, who first described the condition in two infants in 1886.
Today, a number of mouse models are available for colonic aganglionosis, which exploit mutations in the endothelin 3 receptor, as well as glial cell line-derived neurotrophic factor (GDNF) and its receptor, tyrosine kinase.5 In addition to colonic aganglionosis and lack of appropriate colonic contractions, these mutations also result in white spotting of the coat, giving rise to the term “lethal spotting mice”. According to the Jackson Laboratory, lethal white spotting mice and similar homozygous “piebald mice” usually die within the third week of life.7
Another “lethal white” syndrome is seen in guinea pigs. While the genetic basis of this disease has not been worked out, the overall picture strongly suggests defective migration of neural crest cells during embryogenesis. Similar to the syndrome seen in overo foals, the condition arises in approximately 25% of guinea pigs born to parents carrying the red “roan” allele. While aganglionosis has not been documented in affected guinea pigs, affected individuals manifest albinism, microopthalmia, cataracts, hypodontia and malocclusion, intestinal malabsorption, and diminished immune responses.10 They are, however, reputed to have excellent personalities.
The thickness of the muscular tunics was a subject of spirited discussion, with some of the attendees favoring a hypoplastic change due to lack of innervation during development, and some favoring a thinning due to distention from accumulated waste and sampling proximal to the a more profound obstruction.
References:
- Barker I.K., Baker D.C, Brown C.C: The alimentary system. In: Jubb KVF, Kennedy PC, Palmer N eds Pathology of Domestic Animals, 5th ed., vol. 2. Elsevier Saunders, 2007:85-86.
- Kusafuka T, Puri P: Mutations of the endothelin-B receptor and endothelin-3 genes in Hirschsprung's disease. Pediatr Surg Int 1997; 12:19-23.
- Lightbody, T. Foal with overo lethal white syndrome born to a registered quarter horse mare. Can Vet J. 1994; 43(9): 715–717
- Metallinos DL et al., “A missense mutation in the endothelin-B receptor gene is associated with Lethal White Foal Syndrome: an equine version of Hirschsprung disease.” Mamm Genome. 1998; 9: 426-31. PMID: 9585428
- Roberts RR, Bornstein JC, Bergner AJ, Young HM. Disturbances of colonic motility in mouse models of Hirschsprung’s disease. Am J Physiol Gastrointest Liver Physiol 2018: 294:G996-G1008.
- Santschi EM, Purdy AK, Valberg SJ, Vrotsos PD, Kaese H, Mickelson JR. Endothelin receptor B polymorphism associated with lethal white foal syndrome in horses. Mamm Genome 1998; 4:306–309
- The Jackson Laboratory Mouse strain Datasheet 000262 - https://www.jax.org/strain/000262
- Vrotsos P.D., Santschi E.M, Mickelson J.R: The Impact of the Mutation Causing Overo Lethal White Syndrome on White Patterning in Horses. Proceedings of the annual convention of the American association of equine practitioners. 47:385-391
- Webb A.A., Cullen C.L. Coat color and coat color pattern-related neurologic and neuro-ophthalmic diseases. Can Vet J. 2010 Jun; 51(6): 653–657.
- Williams, BH. Non-infections diseases in guinea pigs. In: Suckow MA, Stevens KA, Wilson RP, eds. The laboratory rabbit, guinea pig, hamster, and other rodents. London, Academic Press, 2012, p. 686-702.