Wednesday Slide Conference, 2025-2026, Conference 8, Case 1
Signalment:
1.7-year-old, female spayed, domestic shorthair catHistory:
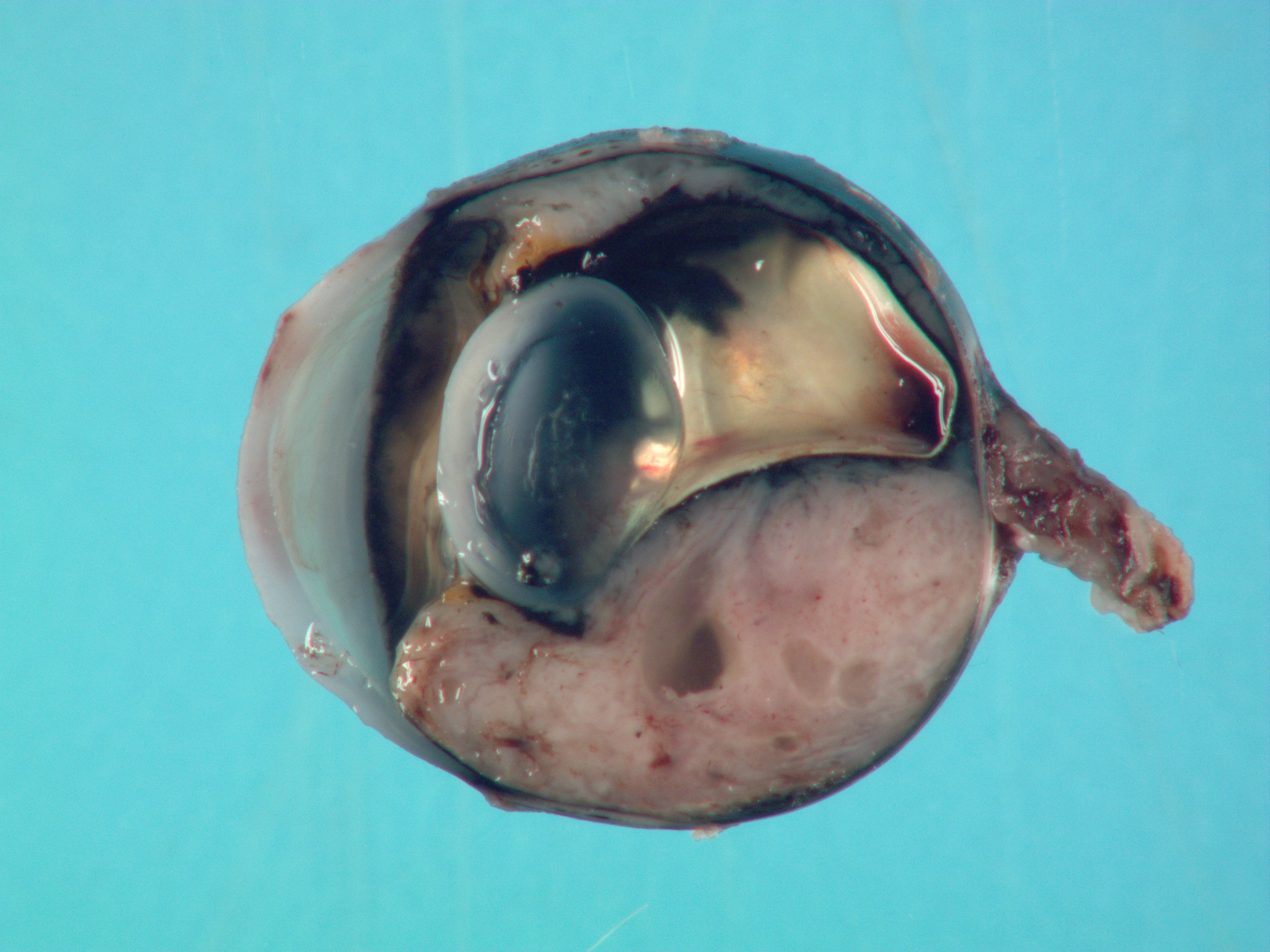
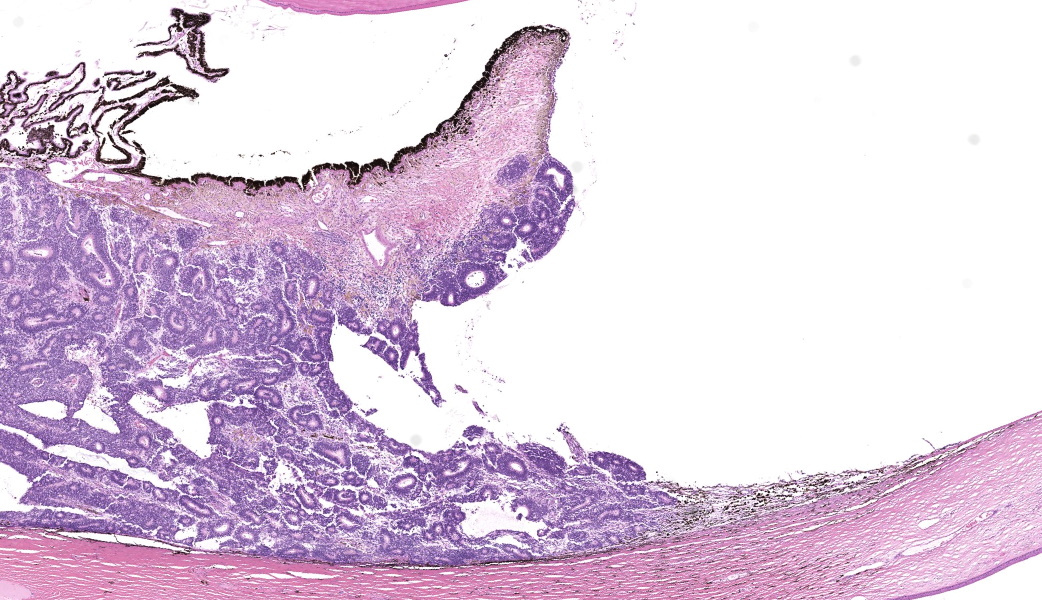
Gross Pathology: On external examination, the eye is notably buphthalmic. The cornea is centrally ulcerated and opaque. On cut surface, a tan, soft mass with multifocal cystic spaces extends from the ventral choroid, protrudes into the anterior and posterior chambers, and abuts and minimally displaces the lens dorsally. The ventral iris is not observed.Laboratory Results:
N/AMicroscopic Description:
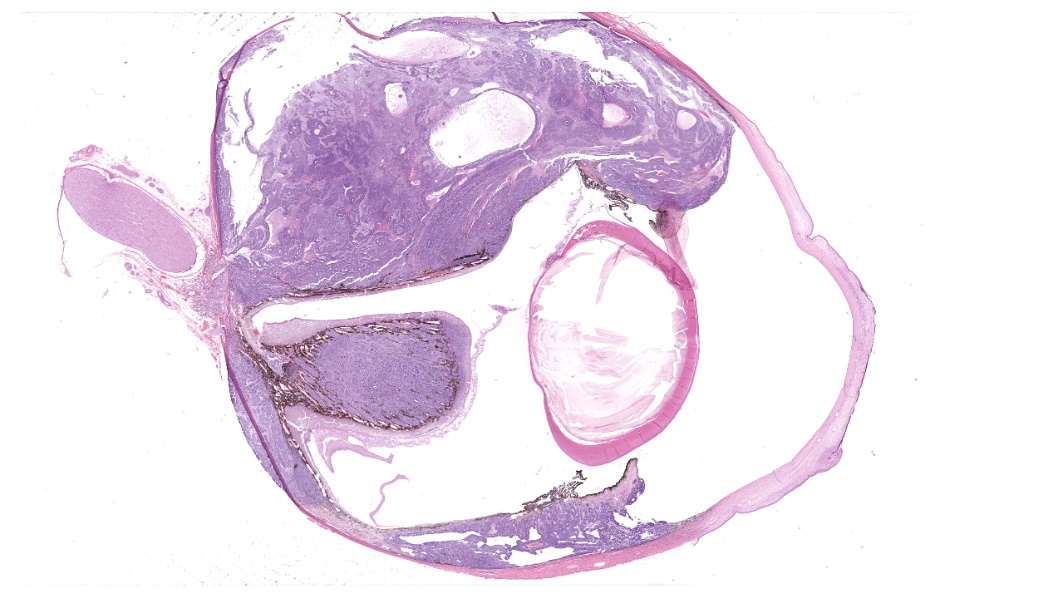
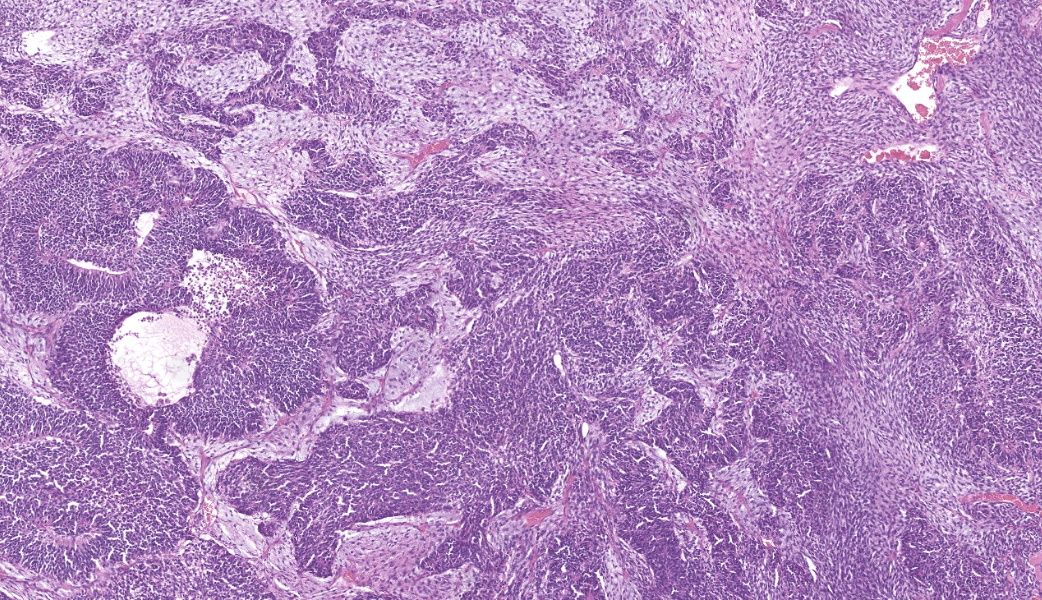
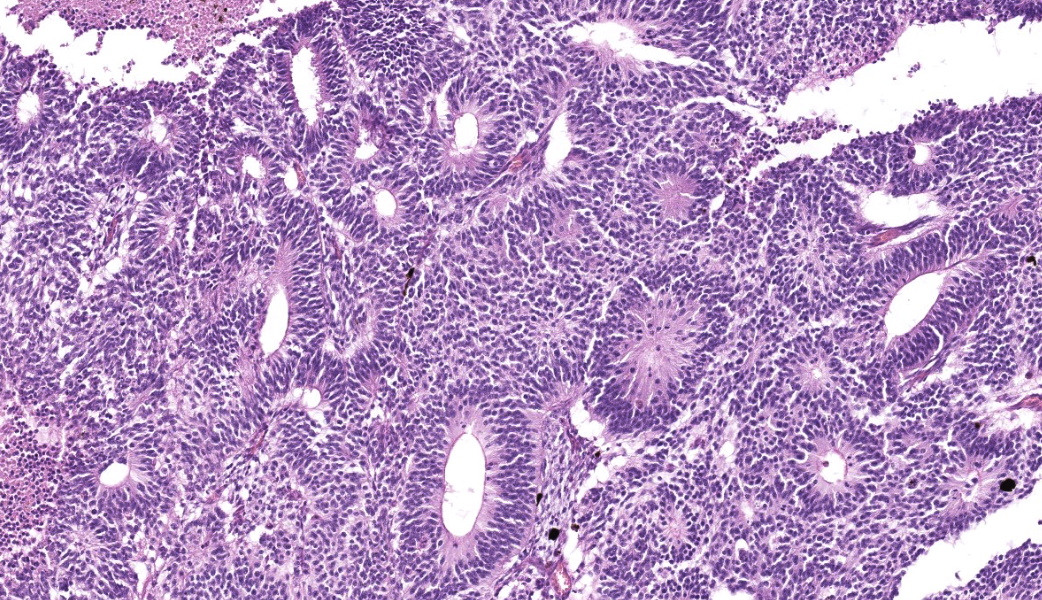
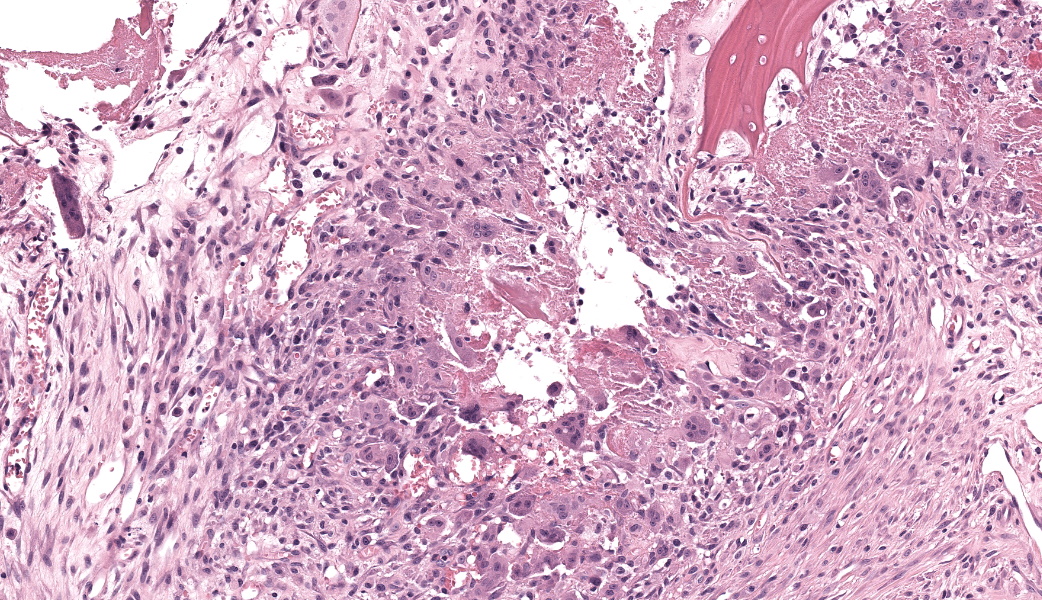
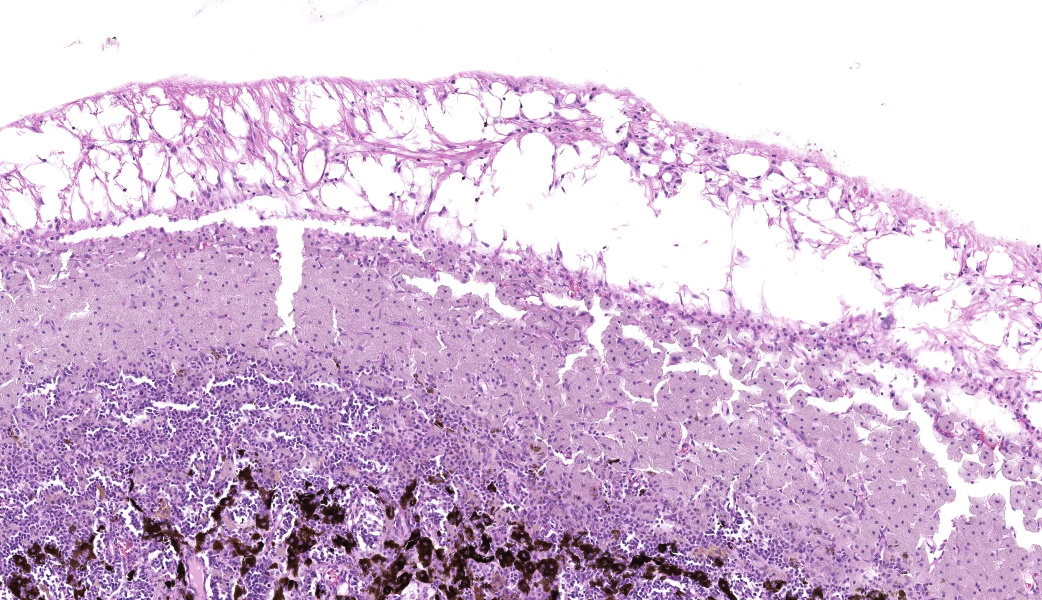
Expanding the anterior uvea, effacing the iris, and markedly expanding the suprachoroidal space is a densely cellular, unencapsulated neoplasm of neuroepithelial cells. Neoplastic cells are arranged in variably dense sheets and as palisading columnar cells surrounding a central lumen (Flexner-Wintersteiner rosettes) or surrounding eosinophilic fibrillar material (Homer-Wright rosettes) surrounded by collagenous and occasionally myxomatous stroma. Neoplastic cells are polygonal to fusiform, have a high nuclear to cytoplasmic ratio, and have distinct cell borders. The nuclei are round to ovoid, finely stippled, and have indistinct nucleoli. There are 16 mitoses in 10 high power fields and anisokaryosis is mild. The neoplastic cells are impinging upon the optic nerve, which has multifocal axonal degeneration and is infiltrated by a population of histiocytes, lymphocytes, and plasma cells, as well as scattered foci of hemorrhage. The retina has multifocal, disorderly components of viable photoreceptor and retinal ganglion cells, dense necrosis, glial scarring, and presumed Mueller cells. The retina is diffusely detached and lacks apparent retinal vessels. The retinal pigment epithelial cells have multifocally migrated through to the inner layers of the retina. The cortical lens fibers are moderately liquefied and homogenous and there is posterior migration of the lens epithelium, consistent with cataractous change. The corneal epithelium has marked, segmental ulceration and the outer corneal stroma in this area is mineralized with occasional breaks and is densely compact with fibrosis. Multifocally throughout the corneal stroma are areas of fibrosis, vascularization, and scattered neutrophils.Contributor's Morphologic Diagnoses:
Eye: Medulloepithelioma, Cataractous change, Ulcerative and neutrophilic keratitis, Stromal fibrosis and mineralization, Diffuse, severe retinal atrophy, Suspect avascular retina, Moderate optic nerve degeneration and necrosis.Contributor's Comment:
Medulloepitheliomas are congenital neoplasms classified as primitive neuroectodermal tumors. They are derived from embryonal neural tissue, the tissue that forms the inner layer of the neural cup. Medulloepitheliomas are categorized into teratoid and nonteratoid forms and further categorized into benign and malignant.8The most common presentation for this tumor is a young animal with a clinically identified intraocular mass. Despite their embryological etiology, medulloepitheliomas have been identified in older animals and age alone is not a disqualifying feature. They have been described primarily in dogs and horses and rarely in other species, including cats, rabbits, and llamas.2,3,4
Medulloepitheliomas classically present grossly as white to tan masses that fill the posterior chamber. The hallmark feature of primitive neuroectodermal tumors is the formation of rosettes. This tumor forms both Flexner-Wintersteiner rosettes (characterized by the formation of a central lumen) and Homer-Wright rosettes (characterized by the lack of central lumen and may surround eosinophilic fibrillar material).10 Importantly, the formation of both rosettes is found in other primitive neuroectoderm tumors and is not unique for medulloepitheliomas. Retinoblastoma is a similar primitive neuroectodermal tumor and must be differentiated histologically from medulloepitheliomas. In retinoblastomas, although they may occasionally have both types of rosettes, Flexner-Wintersteiner rosettes are often the dominant type. While retinoblastomas are common ocular tumors in young children, there are very few accounts of this diagnosis in veterinary species.5 Characteristically, rosettes in a medulloepithelioma are often large, multi-layered and lined by cuboidal cells arranged in a right angle to the lumen. There is pronounced neuroepithelial tubule formation, a distinguishing characteristic not found retinoblastomas. Medulloepitheliomas have been described more robustly in veterinary species, and the presence of large, multilayered, Flexner-Wintersteiner and Homer-Wright rosettes ultimately led to the diagnosis of medulloepithelioma in this case.
Medulloepitheliomas are classified as benign or malignant. Criteria for malignancy set forth by Zimmerman et al. include poor differentiation, sarcomatous change, increased mitotic rate or nuclear pleomorphism, and invasion into other structures such as the sclera or uveal stroma.1 In most veterinary cases with continued follow-up, malignancy was rare. In all cases, enucleation is the preferred treatment method.
Contributing Institution:
National Institutes of Health, Comparative, Biomedical Scientist Training Program (CBSTP), 37 Convent Drive, Bldg. 37, Rm. 2002, Bethesda, MD 20892 https://nih-cbstp.nci.nih.gov/JPC Diagnoses:
Globe, choroid, ciliary body, and iris: Medulloepithelioma.JPC Comment:
This week?s conference was moderated by MAJ Kelsey Fiddes, former Training Officer at the Joint Pathology Center. Two of today?s entities (Cases 1 and 2) are new to the Wednesday Slide Conference and provided excellent discussion amongst participants. The conference kicked off with a review of the gross orientation of the eye and how to determine which aspect of the eye contains a lesion (i.e. dorsal, ventral, lateral, medial, etc. A few key features to assess for orientation are the position of the optic nerve, which tends to exit the eye in a ventromedial direction the location of the tapetum lucidum, which is located in the dorsal fundus in dogs, the location of the ciliary arteries (located dorsolaterally and ventromedially from the optic nerve), the dorsal oblique muscle , which is located dorsally and exiting medially from the globe, and the ventral oblique muscle, which is ventrolateral on the globe and exits medially.Gross preparation of an eye for histopathology was also reviewed and can be summarized as, for standard eye preparation, a vertical cut perpendicular to the ciliary arteries just offset from the optic nerve will provide the best cut for evaluating both tapetal and non-tapetal sides of the choroid. This is especially important for the evaluation of some disease processes, such as tapetal-sparing glaucoma. However, some species require a horizontally oriented cut to ensure that certain structures, such as the pectin in birds or the fovea and macula in some non-human primates, are included in section.
A review of the most pertinent, ?board-worthy? basics of feline intraocular neoplasms was performed in the form of a ?Jeopardy!? with some feline-centric, pun-based category titles, such as ?Meow-lignancy? and ?Pathology Purr-view.? The major takeaways are: iridociliary tumors can co-express both vimentin and cytokeratin; the most common intraocular malignancy of cats is feline diffuse iris melanoma; neoplastic transformation of lens epithelium, as well as the migration of neoplastic cells to cover the inner circumference of the globe, occurs in feline post-traumatic ocular sarcoma; and the most frequently diagnosed orbital tumor of cats is the feline restrictive orbital myofibroblastic sarcoma.
Lastly, there was much discussion on the extensive secondary changes seen in this eye histologically, including keratitis and panuveitis. It is the opinion of conference participants that these changes were likely secondary to the medulloepithelioma and, as such, favored a traditional JPC morphologic diagnosis in such cases which is limited to the tumor and anatomic locations within the globe. This is not to say that the secondary changes are unimportant or should not be listed, but in true JPC tradition, changes secondary to a tumor are not included in the morphologic diagnosis.
The naming of the medulloepithelioma has a rather storied history. It was first called ?carcinome primitif? by French doctors A. Badel and F. Lagrange in their 1892 article titled, "Carcinome primitif des procès et du corps ciliaire" (meaning ?Primitive carcinoma of the processes and ciliary body?). These two individuals are credited with the first description of this neoplasm and are considered foundational in ophthalmologic and embryologic pathology. In 1904, a doctor named F. Verhoeff renamed it ?teratoneuroma? due to the various tissues seen within the neoplasm in some cases.9 In 1908, Dr. E. Fuchs decided to call it ?diktyoma? after the Greek word ?diktyon?, meaning ?net?, secondary to the interlacing bands of neuroepithelial cells and meshwork of medullary epithelial cords seen histologically.9 It wasn?t until 1931 that the term ?medulloepithelioma? made its way to the scene, coined by Dr. R. Grinker, in reference to the histological resemblance of the tumor to the neuroepithelium of the embryonic neural tube, which is now considered one of the main diagnostic features of this neoplasm.9
Intraocular medulloepitheliomas arise from the primitive medullary epithelium of the inner layer of the optic cup. This structure normally develops into the retina, iris, and ciliary body epithelium. This neoplasm most commonly forms in the non-pigmented epithelium of the ciliary body but can rarely originate from the retina or optic nerve. In dogs and cats, intraocular medulloepitheliomas most often follow the playbook and arise from the ciliary body neuroepithelium, but in horses, they tend to go off-book and originate from the optic nerve head.7 Medulloepitheliomas are classified as either benign or malignant, and teratoid or non-teratoid. The teratoid form, as the name would imply, contains tissues that are not present within the normal eye (e.g., cartilage, bone, brain tissue, or muscle). In the conference case, due to the presence of primitive retinal tissue and neuropil within the neoplasm, conference participants favored a ?teratoid? classification for this neoplasm.
Each of the common locations of the intraocular medulloepithelioma represents places where the closure of the optic cup and choroid fissure (also known as the optic stalk fetal fissure) last occur and where the final differentiation of the last multipotent neuroepithelial progenitor cells happens.6 The optic cup forms from invagination of the optic vesicle, which buds from the developing diencephalon. When the optic vesicle contacts the overlying surface ectoderm, this induces the surface ectoderm to thicken and form the lens placode.6 Both the lens placode and the underlying optic vesicle begin to invaginate. The invaginating optic vesicle collapses in on its internal space, forming a two-layered optic cup. The outer layer of this optic cup develops into the retinal pigment epithelium (RPE), while the inner layer differentiates into the neural retina, eventually forming the light-sensing rods and cones.6 The invagination progresses dorsally to ventrally, creating a gap on the ventral surface of the optic cup and stalk called the optic fissure, which is crucial for the development of the optic nerve and other structures. Mesenchymal tissue, mostly from neural crest cells, enters the developing eye through the optic fissure and forms the hyaloid artery, which provides crucial blood supply.6 The two edges of the optic fissure then fuse together, a process that starts near the optic stalk and moves cranially.6 This closure is critical for normal eye development and generally occurs around day 25 in fetal dogs and day 21 in fetal cats under normal conditions. Failure of the optic fissure to close can result in conditions such as coloboma formation.
Although not considered hereditary, current studies indicate that medulloepitheliomas may be linked to certain genetic mutations that disrupt embryonic development. In some cases, medulloepithelioma formation has been associated with germline mutations in the DICER1 gene, which is involved in microRNA processing (hello, Gen Path).9 Others may feature somatic mutations in the KMT2D gene, which plays an important role in histone methylation.9 These genetic changes interfere with the normal differentiation of primitive neuroectodermal stem cells, causing them to develop into a neoplasm instead of their mature, specialized tissues.
References:
- Broughton WL, Zimmerman LE. A Clinicopathologic Study of 56 Cases of Intraocular Medulloepitheliomas. American Journal of Ophthalmology. 1978;85(3):407-418.
- Eagle RC Jr, Font RL, Swerczek TW. Malignant medulloepithelioma of the optic nerve in a horse. Vet Pathol. 1978;15(4):488-94.
- Langloss JM, Zimmerman LE, Krehibiel JD. Malignant intraocular teratoid medulloepithelioma in three dogs. Vet Pathol. 1976;13(5):343-52.
- Monk CS, Craft WF, Abbott JR, et al. Clinical behavior of intraocular teratoid medulloepithelioma in two-related Quarter Horses. Vet Ophthalmol. 2017;20(6):551-559.
- Peiffer RLJr, Simons KB. Ocular Tumors in Animal and Humans. Iowa State University Press, 2002, 1st Edition.
- Rosen D, Mahabadi N. Embryology, Optic Cup. [Updated 2023 May 1]. StatPearls. Treasure Island (FL): StatPearls Publishing; 2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545150/.
- Salih A, Moore D, Giannikaki S, Scurrell E. Malignant ocular teratoid medulloepithelioma in two cats. J Comp Pathol. 2023;201:10-12.
- Saunders T, Margo CE. Intraocular medulloepithelioma. Arch Pathol Lab Med. 2012;136(2):212-216.
- Tadepalli SH, Shields CL, Shields JA, Honavar SG. Intraocular medulloepithelioma - A review of clinical features, DICER 1 mutation, and management. Indian J Ophthalmol. 2019;67(6):755-762.
- Wippold FJ 2nd, Perry A. Neuropathology for the neuroradiologist: rosettes and pseudorosettes. AJNR Am J Neuroradiol. 2006;27(3):488-92