Wednesday Slide Conference, Conference 16, Case 2
Signalment:
Less than 1 year-old female Texel ewe lamb (Ovis aries).
History:
Animal found dead with greenish serous nasal discharge and frothing. No previous clinical signs were reported by the owner.
Gross Pathology:
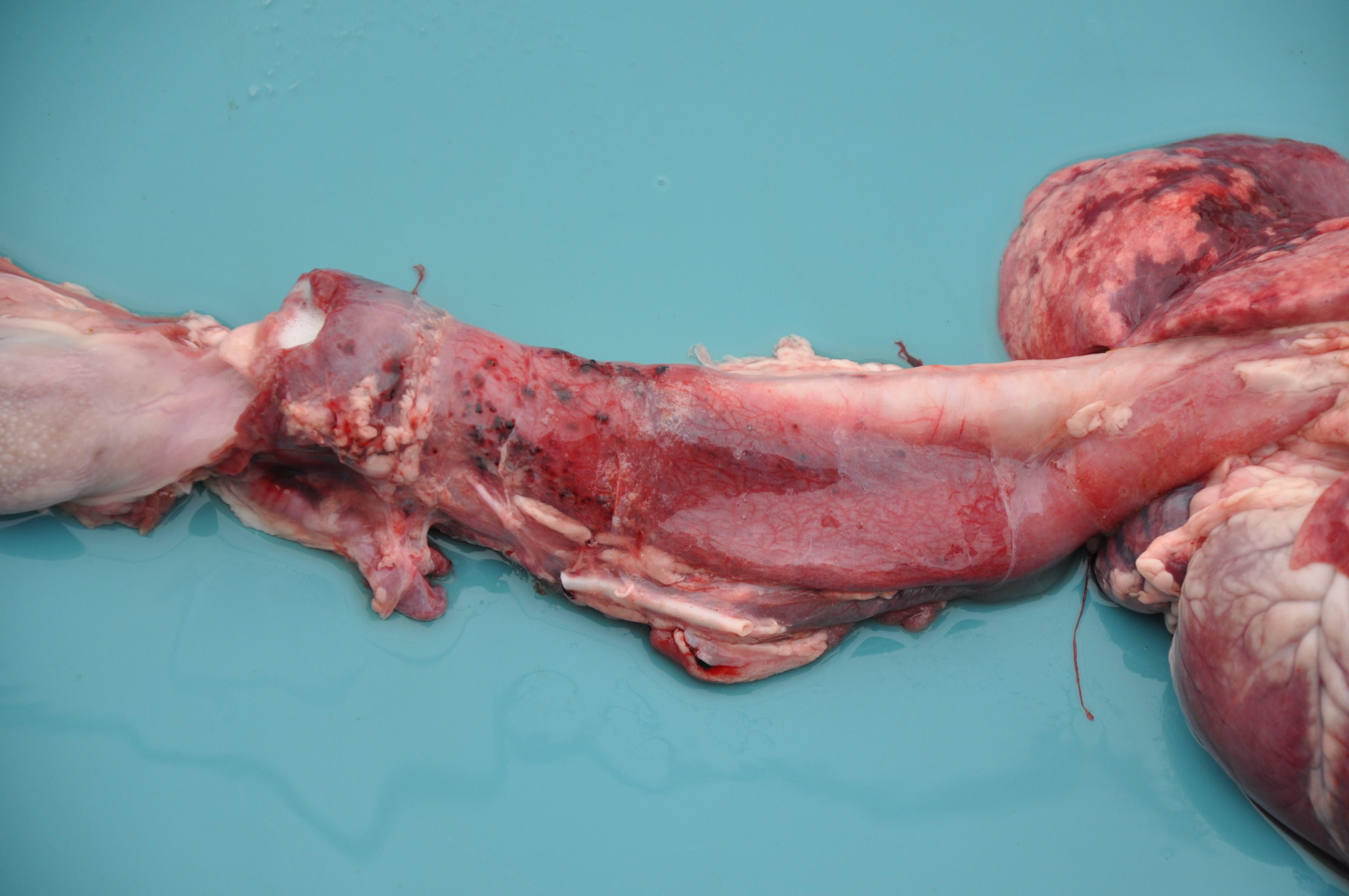
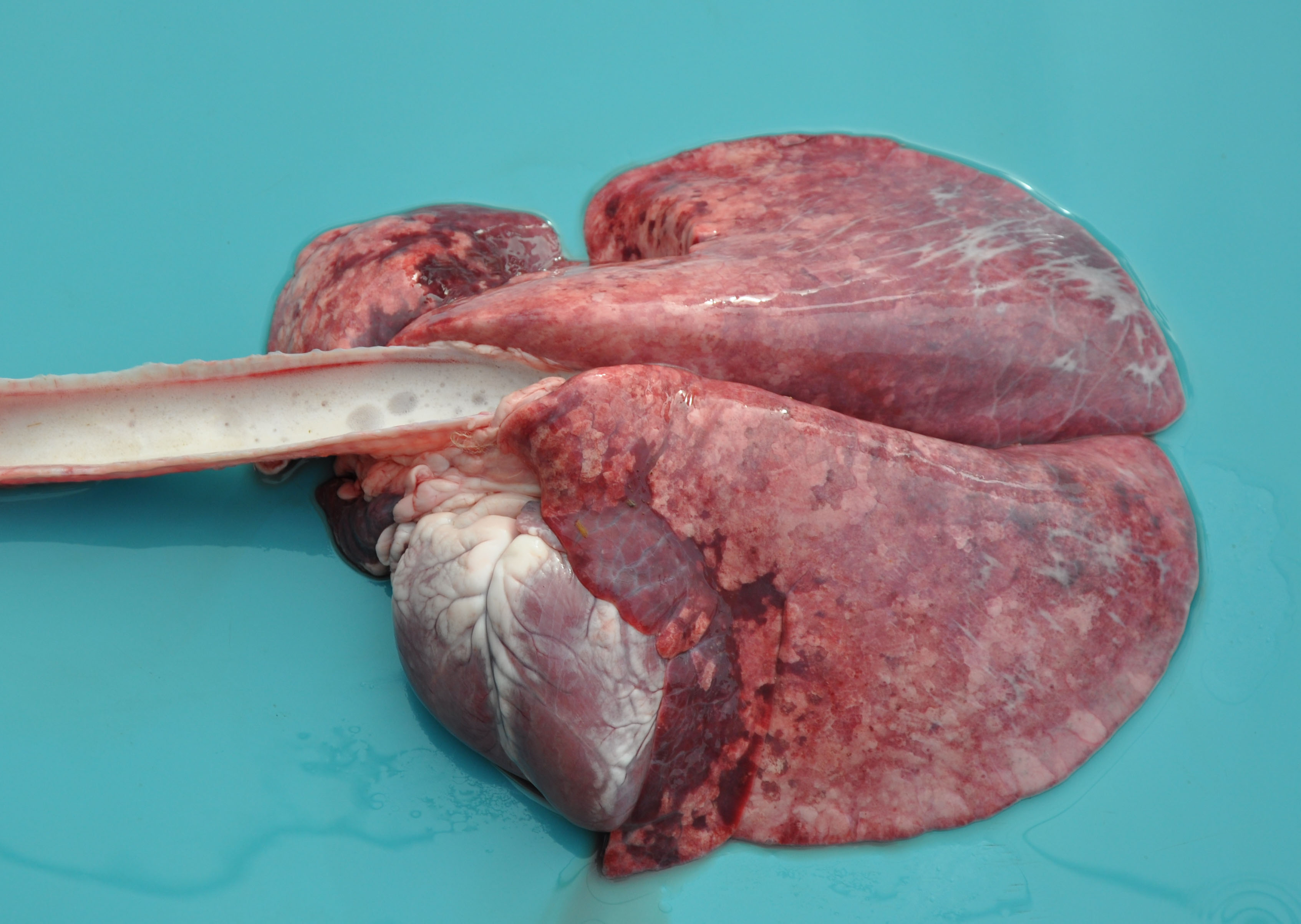
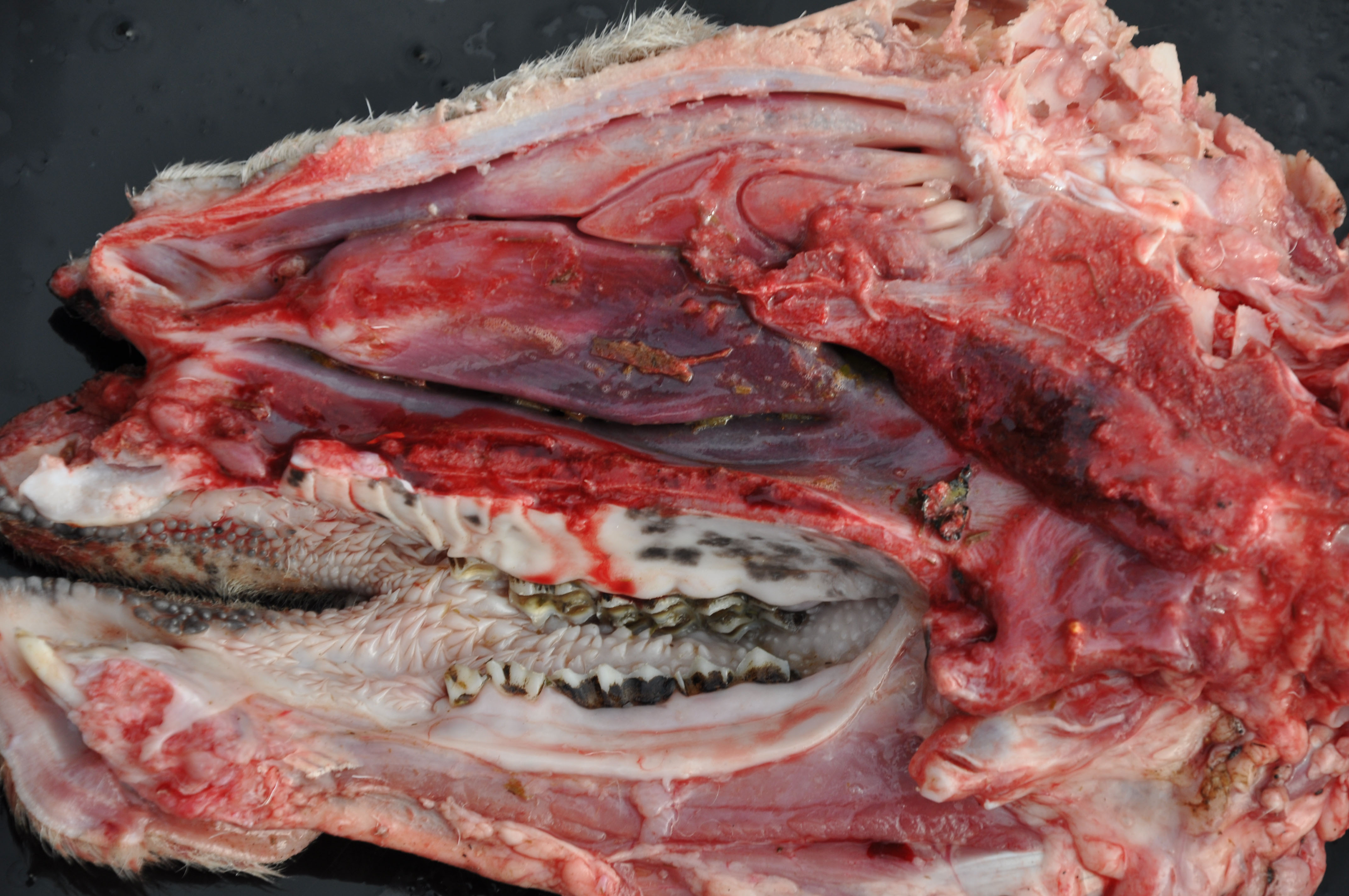
A female Texel ewe lamb was submitted to necropsy examination presenting good body condition and pale ocular mucosa. In nasal planum, a large amount of greenish serous nasal secretion was noticed. Submandibular and retropharyngeal lymph nodes presented moderate enlargement and showed diffuse dark red coloration. In the nasal cavity, moderate amount of inert plant fibers and ruminal content were seen and turbinates were diffusely hyperemic. The thoracic cavity contained a small amount of translucent liquid (hydrothorax). The lungs were not collapsed showing elastic consistency and abundant amount of foamy liquid in bronchi and trachea associated with inert plant fiber, as well as multifocal areas of consolidation found predominantly in the cranioventral region of the lungs. In the cervical segment of the esophagus moderate dilatation and sagging were noticed; the adventitia presented a moderate diffuse coloration with multifocal pinpoint hemorrhages smaller than 1 cm (petechiae). Moderate hydropericardium and multifocal areas of hemorrhage in epicardium were noted. In addition, discrete amount of hairlike parasites compatible with Haemonchus contortus in the abomasum
Laboratory Results:
Blood and tissue samples were tested for BTV RNA detection by RT-qPCR. Bacterial analysis was performed in lung tissue and Mannheimia sp. was isolated.
Microscopic Description:
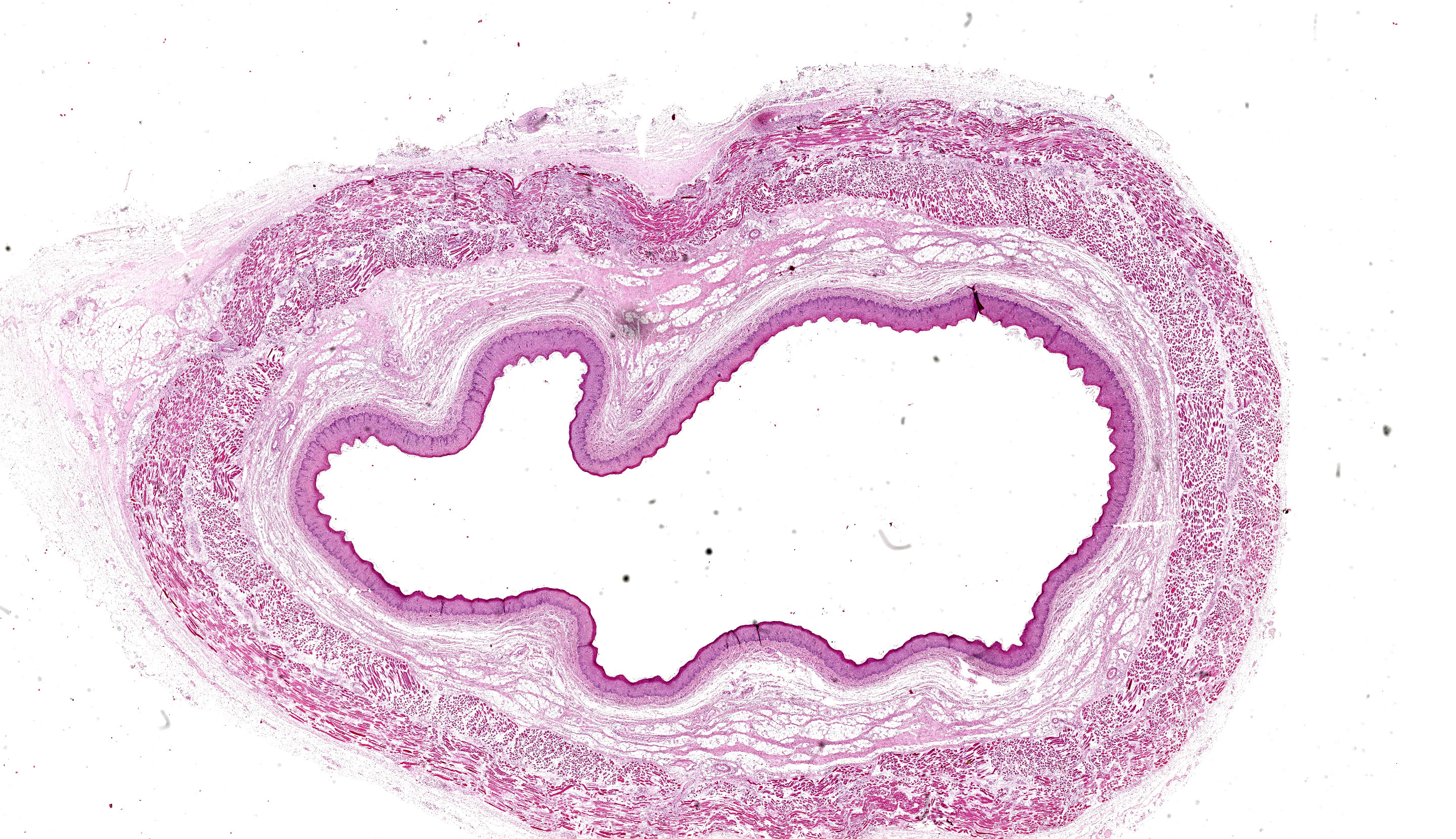
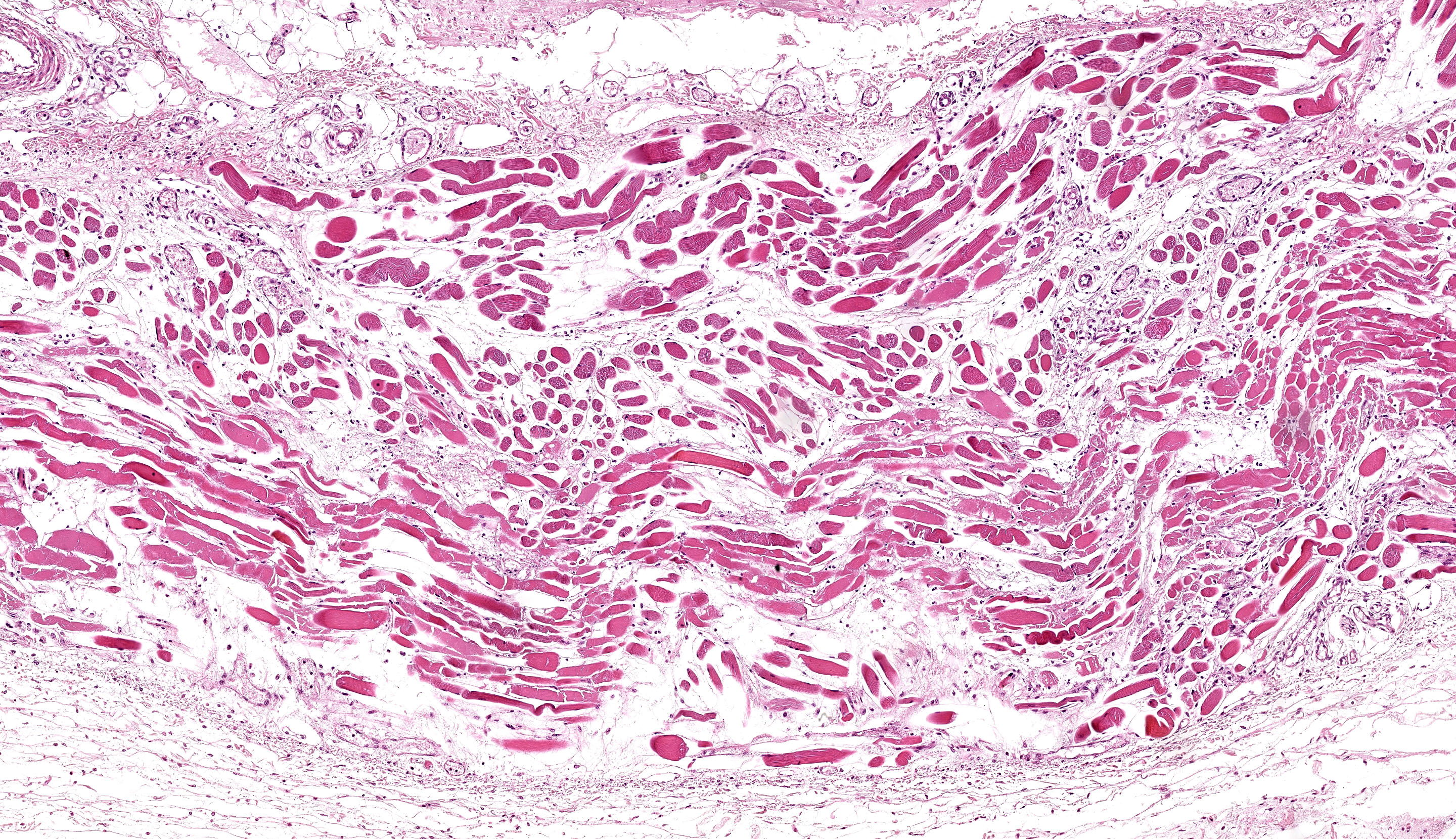
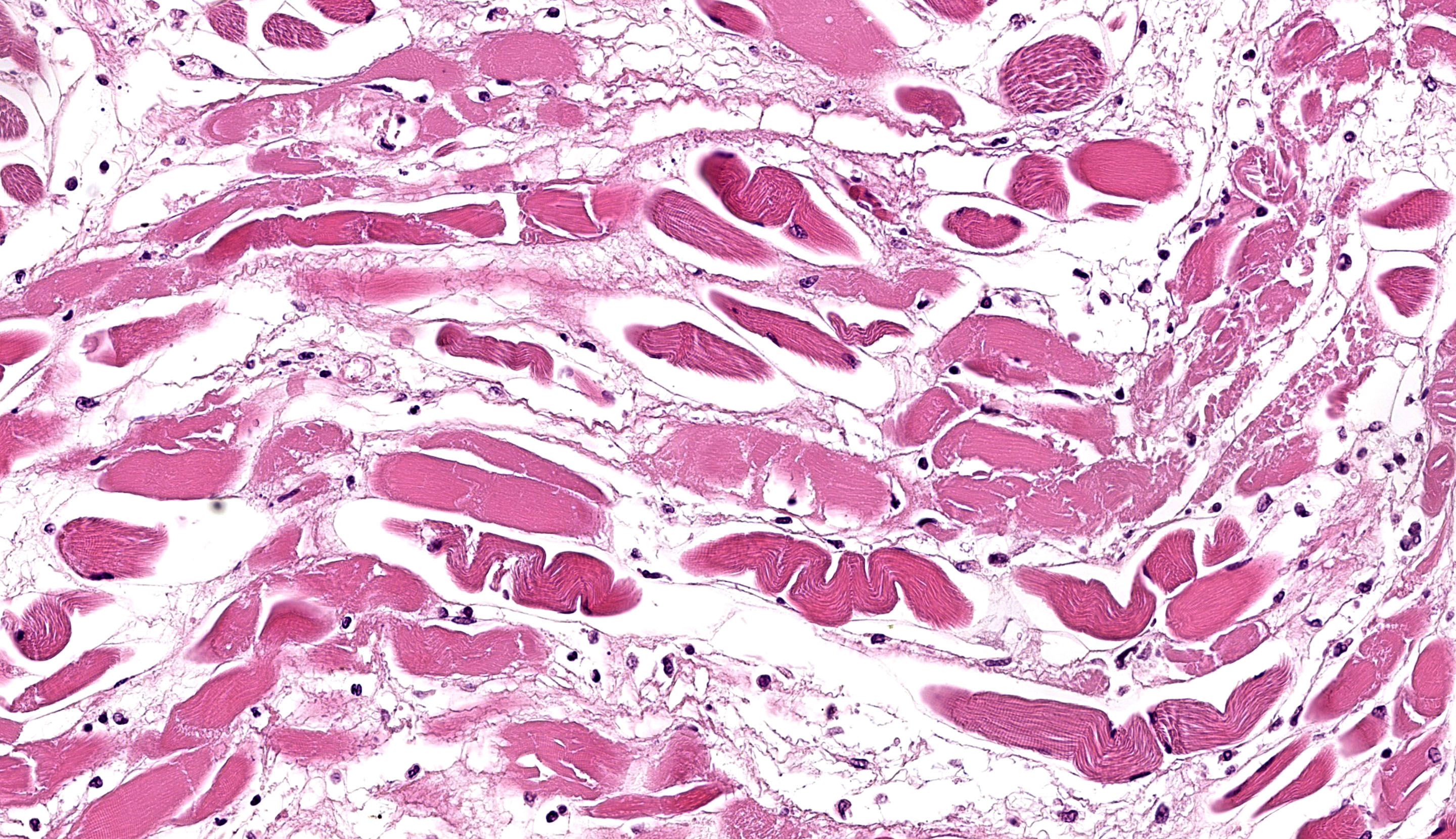
A section of esophagus is examined. In muscular layer, there is severe multifocal to coalescing hyaline and flocculate degeneration and necrosis. Hyaline degeneration and necrosis are characterized by hypereosinophilic and swollen myofibers, with rounded edges
in cross-section, sometimes showing hypercontracted and segmented cytoplasm with loss of striations, as well as pyknotic nuclei; eventually, myocytes display fragmented and flocculate sarcoplasm (flocculate degeneration and necrosis). Moreover, multifocally, macrophages are noted infiltrating cell sarcoplasm, as well as regenerating myofibers, which are characterized by elongated muscle cells with a row of central closely spaced nuclei containing myoblasts. In addition, moderate to severe inflammatory infiltrate of macrophages, lymphocytes, and few neutrophils, edema as well as moderate multifocal congestion were associated with necrotic areas and mild fibroblast proliferation. In some sections, marked multifocal to coalescing areas of hemorrhage are observed in tunica adventitia. Thin-walled structures consistent with Sarcocystis sp. were observed in some slides.
Contributor’s Morphologic Diagnosis:
Esophagus: severe diffuse subacute necrotizing esophagitis.
Contributor’s Comment:
The gross and microscopic findings observed in the present case were compatible with bluetongue disease, which was confirmed by detection of BTV RNA through RT-qPCR. Bluetongue virus (BTV) is a non-enveloped arbovirus, a member of the Reoviridae family, and is the prototype of the genus Orbivirus.1 Bluetongue (BT) is a hemorrhagic disease caused by BTV, which affects domestic and wild ruminants. Among domestic species, sheep are the most susceptible and the severity of clinical signs can vary according to breed, age, and immune status of the affected flock.2 In cattle, bluetongue is hardly noticed6 though cattle are defined as amplifiers and reservoir hosts.8 Virus replication in endothelial cells of small vessels results in distinct disease findings associated with vascular injury, such as tissue infarction, hemorrhage, vascular leakage, and edema.2
BTV is a vector-borne virus, mainly transmitted by biting midges from the genus Culicoides. South America has ideal climatic conditions for the survival and proliferation of Culicoides spp., and as reported by indirect evidence (serological investigations), BTV has spread since 1978 all over the American continent, with the exception of Uruguay.3 BT cases are likely underreported due to the presence of very mild clinical signs, which can be mistaken for other similar endemic diseases.3
Typical signs in affected sheep include pyrexia, facial edema, ocular and nasal discharge, crusting of the muzzle, dyspnea, oral erosions and ulcers, coronitis, lameness and weakness.2 The main gross findings described by Antoniassi et al4 were esophageal dilation associated with non-collapsed enlarged lungs and foamy fluid within the trachea and bronchi, occasionally mixed with ruminal content. Hydropericardium, pale areas in the myocardium, and hemorrhagic foci scattered in the endocardium, epicardium and at the base of the pulmonary artery were also reported, as well as hyperaemia and erosions in the oral mucosa, and subcutaneous edema of the face. Aspiration pneumonia in BTV infection is related to aspiration of rumen content after reflux episodes resulting from severe injury of the esophageal muscles4,5 which leads esophageal paralysis.6 The disease is named bluetongue due to the fact that the tongue may become edematous, congested or cyanotic6 though this gross lesion was not seen in the present case.
Histologically, edema, hemorrhage and microvascular thrombosis can be seen in areas with macroscopic lesions in acute presentations. These microvascular lesions are related with muscular necrosis.6 Bianchi et al7 described that the most common lesions found were located in lungs, esophageal striated muscle, cardiac and skeletal muscles, primarily in the neck and forelimbs.
The differential diagnosis for BTV in sheep should include foot-and-mouth disease, contagious ecthyma, sheep pox,8 photosensitization, and peste des petits ruminants.6 Vitamin E and selenium deficiency may be considered, once muscular necrosis associated with mineralization may resemble the changes observed in nutritional muscular dystrophy (NMD).8 Another orbivirus to take in consideration is the Epizootic Hemorrhage Diseases Virus (EHDV), that may occasionally be responsible to mild clinical signs that resemble BTV in sheep.6
BTV 12,5 BTV-1, BTV-4 and BTV-179 have been described in previous outbreaks in the State of Rio Grande do Sul. Until the moment, the genotyping of the virus responsible for this case has not been performed.
Contributing Institution:
Setor de PatologiaVeterinária, Universidade Federal do Rio Grande do Sul, Brazil (http://www.ufrgs.br/patologia/).
JPC Diagnosis:
Esophagus, muscularis: Degeneration and necrosis, monophasic, diffuse, moderate, with edema.
JPC Comment:
This second case prompted spirited discussion among conference participants. The contributor lays out a detailed summary of bluetongue with characteristic histologic features to look for, though these are challenging to confirm in this section. Although there is notable edema and myocyte degeneration and necrosis, the cause is not apparent. We did not identify vasculitis and/or thrombosis in this particular section which would be expected in this case, as BTV is an endotheliotropic agent.
Conference participants offered ionophore toxicity as a potential etiology in this case, which is a ruleout for monophasic muscular injury in small ruminants. Vitamin E/selenium imbalance would likely result in polyphasic injury which would result in additional lesions of mineralization and fibrosis, which are lacking in this case. Early degenerative changes are present in myocytes, to include the early reversible change of hyalinization and hypereosinophilia resulting from glycogen depletion (and lack of replenishment). We covered monensin and associated muscular changes in a recent WSC in a brahman calf (Conference 5, Case 3, 2024-2025).
Our diagnosis is different from the contributor in this case. We framed this case as necrosis and degeneration (vice myositis) due to the lack of significant inflammation this section. In this case, the main driver of the lesion is the presumed vasculitis and ischemic damage – inflammation is mild at best in this lesion. Additionally, the lack of involvement of the esophageal mucosa makes the focus of the lesion more selective, and we prefer to restrict the morphologic diagnosis to the muscular tunics of the esophagus alone.
References:
- Mertens PPC, Diprose J, Maan S, Singh KP, Attoui H, Samuel AR. Bluetongue virus replication, molecular and structural biology. Vet Ital. 2004; 40:426–437.
- Maclachlan NJ, Drew CP, Darpel KE, Worwa G. The pathology and pathogenesis of bluetongue. J Comp Pathol. 2009; 141:1–16.
- Lobato ZIP, Guedes MIMC, Matos ACD. Bluetongue and other orbiviruses in South America: gaps and challenges. Vet Ital. 2015; 51:253–262.
- Antoniassi NAB, Pavarini SP, Ribeiro LAO, Silva MS, Flores EF, Driemeier D. Alterações clínicas e patológicas em ovinos infectados naturalmente pelo vírus da língua azul no Rio Grande do Sul. Pesq Vet Bras. 2010; 30: 1010–1016.
- Antoniassi NAB, Pavarini SP, Henzel A, Flores EF, Driemeier D. Aspiration pneumonia associated with oesophagealmyonecrosis in sheep due to BTV infection in Brazil. Vet Rec. 2010; 166: 52–53.
- Uzal FA, Plattner BL and Hostetter JM. Alimentary System In: Maxie MG, ed. Jubb Kennedy and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:1-257.
- Bianchi RM, Panzieira W, Faccin TC, et al. Clinical, pathological and epidemiological aspects of outbreaks of bluetongue disease in sheep in the central region of Rio Grande do Sul. Pesq Vet Bras. 2017; 37(2):1443-1452.
- Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Veterinary Medicine. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats. 10th ed. Philadelphia, PA: Saunders Elsevier; 2007:1299-1305.
- Guimarães LLB, Rosa JCC, Matos ACD, et al. Identification of bluetongue virus serotypes 1,4, and 17 co-infections in sheep flocks during outbreaks in Brazil. Res Vet Sci. 2017; 113:87-93.