Signalment:
Gross Description:
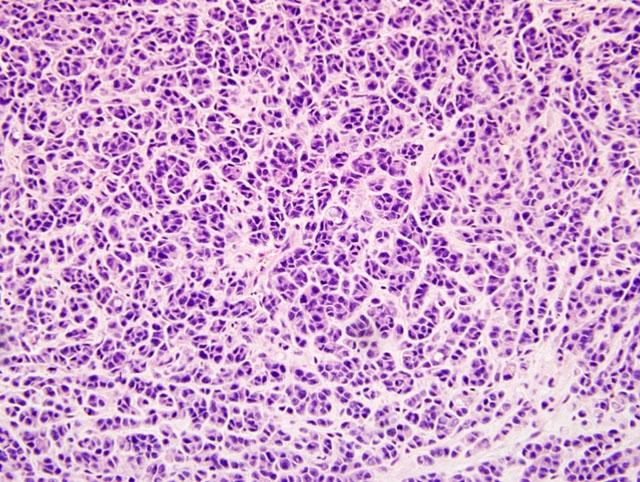
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
- Significant clinical chemistry abnormalities included:
- AST (IU) 436 (39.0-206.0)
- Creatinine (umol/L) 28 (44.0-106.0)
- Protein (g/L) 75.6 (52.0-69.0)
- Albumin (g/L) 24.3 (28.0-37.0)
- Globulin (g/L) 51.4 (18.0-37.0)
- Hematological abnormalities included:
- WBC (x 109/L) 59.5 (3.4-13.2)
- Neutrophils (x 109/L) 55.93 (0.82-9.33)
- Monocytes ((x 109/L) 1.78 (0.04-0.26)
- Fibrinogen (g/L) 12.0 (0.0-3.0) Urinalysis
- Volume (ml) 10
- Color yellow
- Turbidity clear
- Specific Gravity 1.058
- pH 7.0
- Protein 3+
- Glucose nil
- Ketones nil
- Bilirubin nil
- Blood (heme) 2+
- Urobilinogen nil
- Cells (per hpf) 0
- Debris 1+
Comment:
The serum biochemistry changes indicate a moderate increase in AST, moderate decrease in Creatinine, mild hyperproteinemia characterized by a mild hypoalbuminemia and a moderate hyperglobulinemia. The leukon changes demonstrate a marked leukocytosis characterized by a marked neutrophilia and a moderate monocytosis. There is a marked hyperfibrinogenemia, moderate hemoglobinuria, and marked proteinuria. The increase in AST in this case could be attributed to a myopathy related to exertion or capture, since the hepatic function was normal. Although CK was within the normal reference range, AST can remain elevated for up to 5-6 days once released from the muscle, whereas CK can diminish rapidly (within 2 days). Moreover, a delay in sample collection may result in there being no apparent change in activity of CK. The decrease in serum creatinine can occur with severe cachexia and subsequent significant loss of muscle. Low albumin could be due to decreased intake (starvation) or reduced synthesis to maintain oncotic pressure (since globulins are increased). The hypoproteinemia and hyperglobulinemia are consistent with neoplasia and chronic inflammatory disease. The leukon changes (marked neutrophilia, moderate monocytosis and hyperfibrinogenemia) are consistent with severe inflammatory disease.
Condition:
Contributor Comment:
Out of all of Australias unique wildlife, Tasmanian Devils are the largest living dasyurids, or carnivorous marsupials. They are iconic natives of Tasmania, where they inhabit the coastal forests, scavenging on dead or dying animals. A debilitating disease with proliferating facial masses was detected in wild populations of Tasmanian Devils in the mid 1990s.(1,5) Dubbed the Devil Facial Tumour Disease, this emerging new disease was quickly increasing in prevalence, with population declines of up to 80% in some areas.(1,2) Affected animals develop large, multicentric, flat soft tissue masses, often with ulcerative and exudative centers.(2) The tumors first develop around the face, mouth, and neck, but may spread to elsewhere in the body. As they enlarge, the tumors interfere with feeding, and the devils quickly lose condition, and usually succumb to the disease within 6 months.
A multi-disciplinary approach was taken to investigate and characterize this disfiguring, fatal disease. The main goal of this research was to maintain an ecologically sustainable population of Tasmanian Devils in the wild.(5) Standard cytology, histopathology, and electron microscopy were used to characterize these facial tumors. Loh et al (2006a) discovered that the neoplasm originated within the dermis, and was composed of dense, multinodular proliferations of pleomorphic round to polyhedral cells, with a high nuclear to cytoplasmic ratio.(2) These cells had a fibrillar, eosinophilic cytoplasm, indistinct cytoplasmic margins, and single, basophilic nuclei with no obvious nucleoli.(2) The mitotic rate ranged from 0-12 per 400x magnification, and necrosis was observed in most samples.( 2) Metastasis was also reported in 65% of the cases, with frequent involvement of the regional lymph nodes, and distant metastases mainly to the lungs, but also the spleen, heart, ovary, serosal surface of ribs, kidney, mammary, pituitary and adrenal glands.(2)
The pleomorphic neoplastic cells were difficult to characterize, and immunohistochemistry was utilized for final confirmation. The cells stained negative for cytokeratin, epithelial membrane antigen, von Willebrand factor, smooth muscle actin, desmin, glial fibrillary acid protein, CD 16, CD 57, CD3, and LSP1, but stained positive for vimentin, S-100, melan A, neuron specific enolase, chromogranin A, and synaptophysin 3. With this information, together with the morphological and ultrastructural features of the cells, it was concluded the neoplasm was consistent with a malignant neuroendocrine tumor.
In parallel with this work, cytogenetic studies were performed, and a unique, aneuploid karyotype was identified, which was shared by tumors from animals in many geographical locations within the state. These genetic rearrangements were identical in male and female animals from a range of ages, indicating that cytogenetically, DFTD is relatively stable.(4) Such findings, combined with the frequent habit of the devils engaging in jaw wrestling and the propensity for the tumors to arise in the lips, oral mucosa, or the face, is consistent with disease being spread as allografts. The most significant confirmative indicator that DFTD is a transmissible neoplasm has been the successful experimental transfer of DFTD cells derived from cell cultures and natural tumors to healthy devils with the subsequent variable development of DFTD. This work has fulfilled elements of Kochs postulates, confirming the transmissible neoplasm hypothesis. (5)
A collaborative effort to save the Tasmanian Devils has been established with the goal to investigate disease control mechanisms and identify management options to prevent further population decline.
JPC Diagnosis:
Conference Comment:
The contributor did an outstanding job of reviewing DFTD including transmission, gross and histologic findings and tumor karyotype.
Additionally, some attendees noted the incidental finding of intrafollicular mites consistent with Demodex in their sections.
References:
2. Loh R, Bergfeld J, Hayes D, OHara A, Pyecroft S, Raidal S, Sharpe R: The pathology of Devil Facial Tumor Disease (DFTD) in Tasmanian Devils (Sarcophilus harrisii) Vet Pathol 43:890-895, 2006
3. Loh R, Hayes D, Mahjoor A, OHara A, Pyecroft S, Raidal S: The immunohistochemical characterization of Devil Facial Tumor Disease (DFTD) in the Tasmanian Devil (Sarcophilus harrisii) Vet Pathol 43:896-903, 2006
4. Pearse AM, Swift K: Transmission of Devil Facial Tumour Disease. Nature. 439:549, 2006
5. Pyecroft SB, Pearse AM, Loh R, Swift, K, Belov K, Fox N, Noonan E, Hayes D, Hyatt A, Wang L, Boyle D, Church J, Middleton D, Moore R: Towards a case definition for Devil Facial Tumour: what is it? Ecohealth Journal Consortium, 2007