Signalment:
The animal was azotemic and hypoglycemic with marked leukopenia and metabolic alkalosis. The animal had not responded to fluid therapy and was euthanized. Candida albicans was isolated from the stomach contents at necropsy.
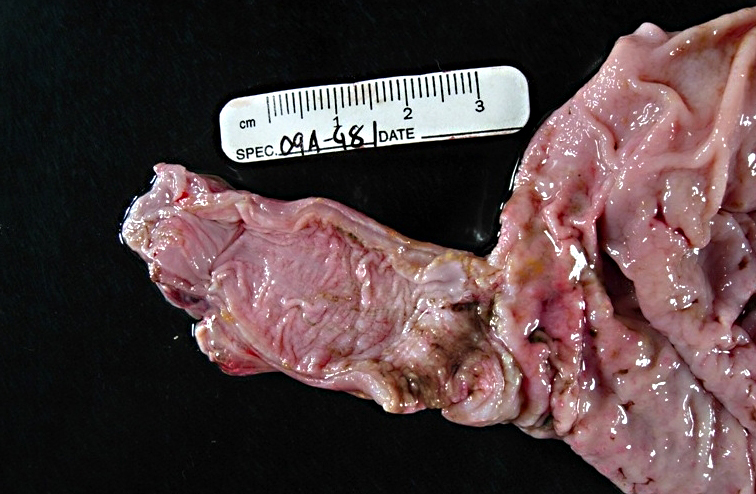
Gross Description:
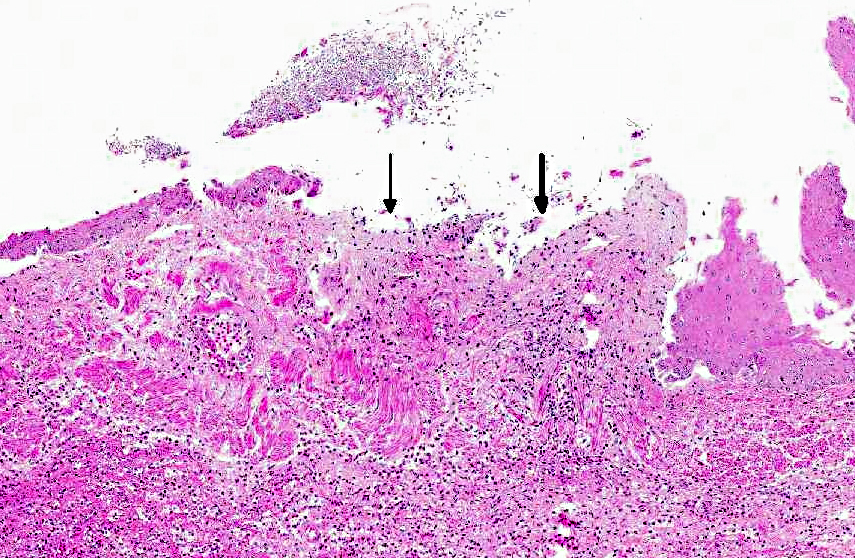
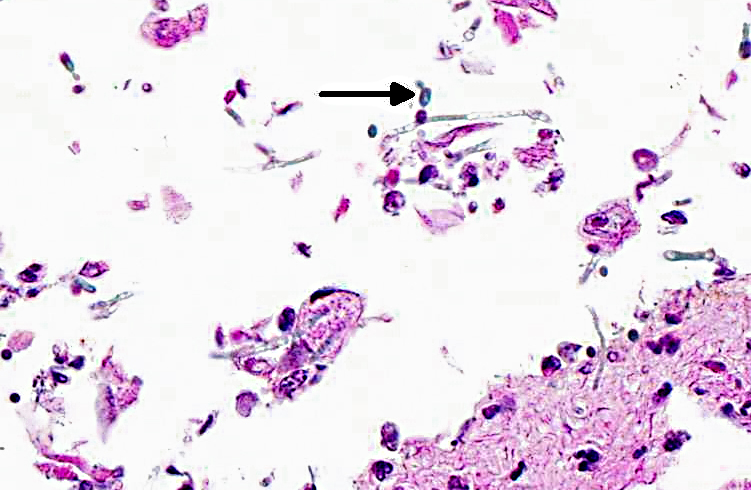
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
| Reference Range | ||
| RBC | 6.37 X106/mm3 | 5.0 - 6.5 X 106/mm3 |
| WBC | 2.8 X103/mm3 | 6.0 - 15.0 X103/mm3 |
| Lymphocytes | 19% | 25.0 - 60 % |
| Monocytes | 1% | 0 8 % |
| Eosinophils | 0% | 0.0 - 5.0 % |
| pH | 7.304 | >7.40 |
| Sodium | 150 mmol/L | 141-153 mmol/L |
| Potassium | 3.0mmol/L | 2.9-4.1 mmol/L |
| Blood Urea Nitrogen | 82mg/dl | 16-27 mg/dl |
| Glucose | 34 mg/dl | 39-82 mg/dl |
Condition:
Contributor Comment:
This animal received multiple intravenous antibiotics following surgical implantation of hormonal depots. The gastritis lesions were chronic and the esophageal lesions are attributed to acid reflux and subsequent colonization by Candida albicans. In rhesus monkeys infected with simian immunodeficiency virus (SIV), candidiasis is a common opportunistic infection; however, this animal has not been infected with SIV. Also, nonhuman primates are an excellent animal model for studying oral candidiasis.(8,9)
Systemic and cutaneous candidiasis has also been described in cattle, calves, sheep, and foals secondary to prolonged antibiotic or corticosteroid therapy.(4) In cats, candidiasis is rare but has been associated with oral and upper respiratory disease, pyothorax, ocular lesions, intestinal disease, and urocystitis. In dogs, C. albicans is reported to cause stomatitis, spondylitis, endophthalmitis and purulent pericarditis.(3,5,6,7)
Candida spp. has been considered a cause of arthritis in horses and mastitis and abortion in cattle. In birds, the infection causes stomatitis, esophagitis and ingluvitis. In piglets, the infection causes stomatitis, esophagitis and gastritis.(1) In horses, C. albicans causes ulcerative gastritis adjacent to margo plicatus.(1)
Candida spp. are pleomorphic, with both yeast and mycelia phases present in tissue. The differential diagnoses include: Aspergillus species., which form septate hyphae with dichotomous branching and bears conidiospores; Zycomycetes species, which form nonseptate, branching hyphae with bulbous enlargement; Histoplasma capsulatum, which are intrahistiocytic yeast; and Blastomyces dermatitidis, which are 7-17 μm large yeast with broad- based budding.
JPC Diagnosis:
Conference Comment:
Pathogenicity of fungal organisms is related to their morphology. In Candida albicans, the single-cell yeast form is thought to be evolutionarily adapted for colonization of mucosal cell surfaces and allows for rapid dissemination via the bloodstream in systemic infections; pseudohyphae are associated with increased virulence properties and enhanced nutrient scavenging; and the formation of hyphae is an important virulence factor which allows the fungus to invade epithelial and endothelial cells and lyse macrophages and neutrophils. The necessity of hyphal formation for pathogenicity is demonstrated by the significant attenuation of virulence in C. albicans cells lacking the filament-induced gene HGC1, which drives hyphal development. In addition, several other hyphalspecific genes are also important for pathogenicity. ALS3 and HWP1 encode adhesins, which allow C. albicans to leave the circulation, colonize tissue, and form a biofilm. Degredative enzymes such as aspartyl proteinase (SAP) contribute to tissue invasion. SOD5, which encodes a superoxide dismutase that protects against oxidative stress, is also induced during hyphal growth. HYR, another hypha-specific gene, plays a role in neutrophil killing. Thus, the ability of C. albicans to form hyphae contributes to their increased virulence compared to other Candida species that only form yeast and pseudohyphae.(10)
References:
2. Brown MR, Thompson CA, Mohamed F. Systemic candidiasis in an apparently immunocompetent dog. J. Vet. Invest. 2005;17:272-276.
3. Jadahav VJ, Pal M. Canine mycotic stomatitis due to Candida albicans. Rev Iberoam Micol. 2006;23:233-234.
4. Jones T, Hunt R, King N. Veterinary Pathology. 6th ed. Baltimore, MD: Williams and Wilkins; 1997:528-529.
5. Kuwamura M, Ide M, Yamate J, Shiraishi Y, Kotani T. Systemic candidiasis in a dog, developing spondylitis. J. Vet. Med. Sci. 2006;68(10):1117-1119.
6. Linek J. Mycotic endophthalmitis in a dog caused by Candida albicans. Vet. Ophthal. 2004;7:159-162.
7. Mohri T, Takashima K, Yamane T, Sato H, Yamane Y. Purulent pericarditis in a dog administered immune suppressing drugs. J. Vet. Med. Sci. 2009;71:669-672.
8. Osborn KG, Prahalada S, Lowenstein LJ, Gardner MB, Maul, DH, Henrickson RV. The pathology of an epizootic of acquired immunodeficiency in rhesus macaques. American Journal of Pathology. 1984;114 (1):94-103.
9. Samaranayake Y, Samaranayake LP. Experimental oral candidiasis in animal models. Clini. Microbio. Rev. 2001;14:398-429.
10. Thompson DS, Carlisle PL, Kadosh D. Coevolution of morphology and virulence in Candida species. Eukaryotic Cell. 2011;10(9):1173-1182.