Signalment:
4-year-old female, Norwegian red,
Bos taurus.The cow was submitted to the large animal clinic at the Norwegian School of
Veterinary Science due to a large (walnut-sized) tumor-like growth in the cornea of the right eye.
A squamous cell carcinoma or a granuloma due to trauma was suspected, and the eye was
enucleated and submitted for histopathologic examination.
Gross Description:
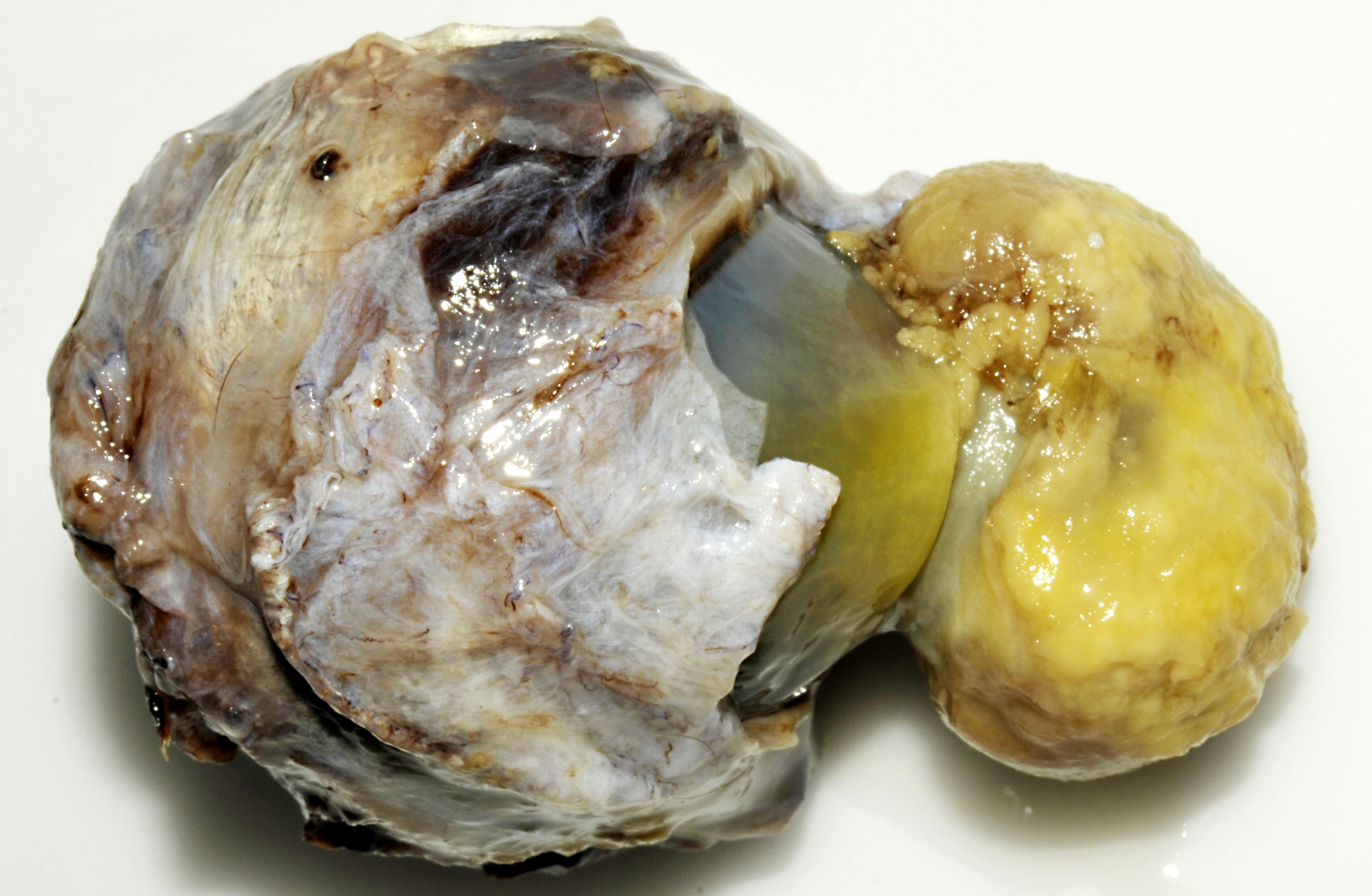
Slightly off the center in the cornea there is a 3x2x2 cm large exophytic
pedunculated nodule with a stalk that measures 1.5 cm in diameter. The surface of the lesion is
rugged with a grey to yellow discoloration. There is opacity of the surrounding corneal tissue.
On the cut surface the lesion appears to be composed of an outward bulging of the cornea with a
central core of gelatinous material. Black pigmented tissue (iris) is adhered to the internal
surface of the cornea and also protrudes into the stalk of the lesion.
Histopathologic Description:
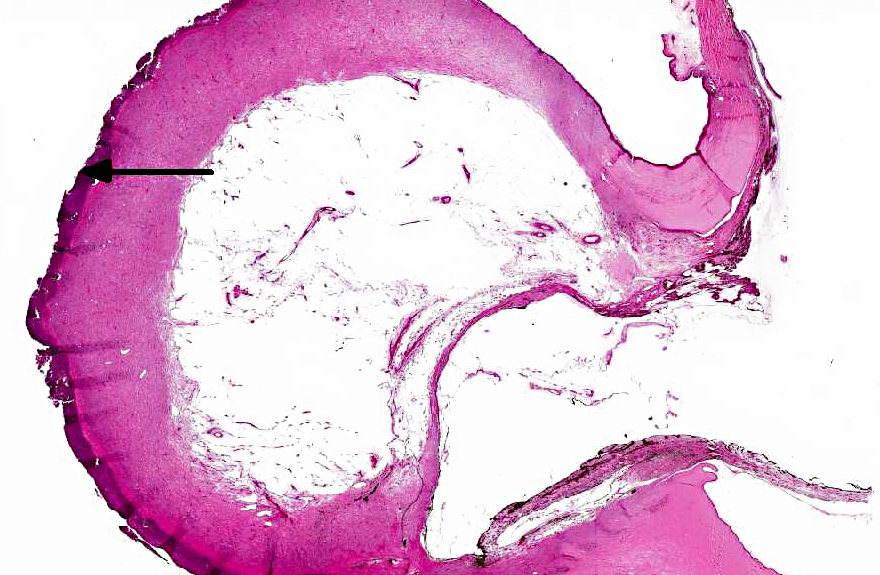
In the eye there is a focally extensive anterior bulging of a
fibrous membrane, and the bulging area consists of connective tissue with collagen fibers parallel
with the surface and vessels that are perpendicular to the surface (granulation tissue). The
granulation tissue is continuous with cornea on each side. The outer surface of the bulging area
is severely inflamed and infiltrated by numerous leukocytes, mainly necrotic neutrophils.
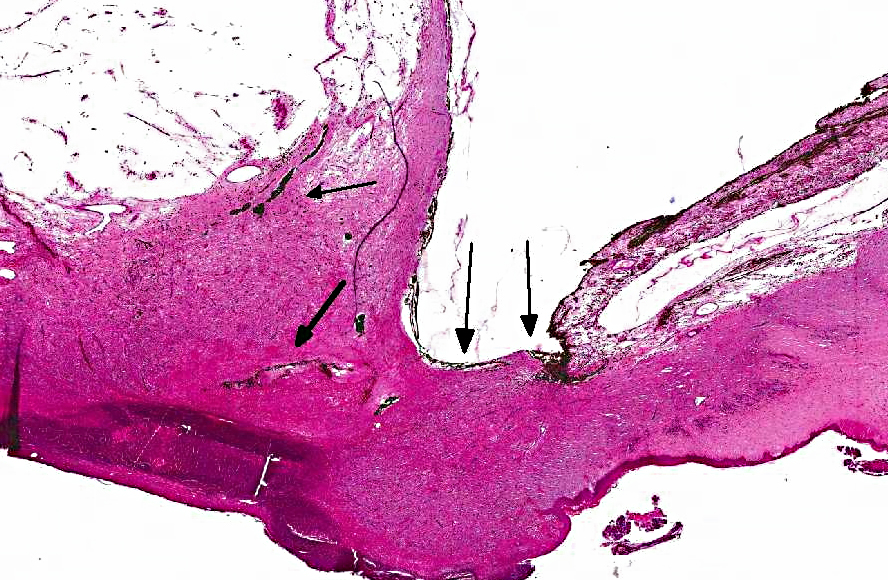
Descemets membrane is ruptured, and the ends are located at each side of the stalk area, thus the
inner surface of the granulation tissue is not lined by Descemets membrane. The center of the
nodule (macroscopic gelatinous area) consists of scattered vessels and spindle shaped cells or
round cells with dark brown pigment, and very few lymphocytes and plasma cells. Towards the
anterior chamber, the lesion is lined by iridal pigmented tissue that is adhered to the cornea
(anterior synechia) and bulging into the defect in the cornea. The pigment in the adhered iris and
the central area was Prussian blue negative, and it was bleached by potassium permanganate
(melanin).
Morphologic Diagnosis:
Eye: keratitis, ulcerative and purulent, focally
extensive, chronic, severe with anterior synechia and corneal staphyloma.
Lab Results:
The intraocular pressure was decreased in the eye.
Condition:
Traumatic staphyloma
Contributor Comment:
The lesion in this eye was interpreted to have been caused by a
traumatic injury to the eye, most likely perforating the cornea, although there was no known
history of trauma. Transcorneal gaps may be initially plugged with fibrin and sometimes by
prolapsed iris.(3) The outward bulging of the cornea in this specimen has the morphology of
granulation tissue, indicating the severity and chronicity of the condition. Although severely
inflamed, the inflammation is mainly present in the superficial layer of the granulation tissue that
was continuous with the cornea. Infectious ulcerative keratitis, e.g. mycotic of bacterial, may
also cause severe corneal lesions. Proteases from microbes or leukocytes may cause stromal
liquefaction (keratomalacia) that may reach Descemets membrane and result in its forward
bulging as a descemetocele.(3) In this case, the Descemets membrane was ruptured with its ends
located at each side of the protruding iridal tissue. Staphyloma is defined as a protrusion of the
sclera or cornea, usually lined by uveal tissue. The lesion can be anterior or posterior and affect
both sclera and cornea. Corneal staphyloma is defined as a bulging of the cornea with adherent
uveal tissue, as was present in this case.(1) Between the granulation tissue and the protruding
iridal tissue there was a large area with scattered blood vessels and pigmented cells. The
pigment was negative in Prussian blue staining, however it was bleached in potassium
permanganate treated slides, indicating that these cells contain melanin pigment.
JPC Diagnosis:
Cornea: Keratitis, ulcerative, chronic, focally extensive severe with staphyloma.
Conference Comment:
Corneal injury, such as was suspected in this case, often results in
alterations of normal healing that disrupt the normal structure and interfere with the function of
corneal tissue.(2) The cornea, which covers the anterior portion of the globe, serves to both protect
the intraocular structures and to refract light for vision. Therefore its most important feature is
its transparency, which is achieved by its architecture. The cornea is composed of three layers
(epithelial, stromal and endothelial layers) and two acellular layers (Bowmans layer and
Descemets membrane, which separate the epithelium from the stroma and the stroma from the
endothelium, respectively). These layers have a uniform, consistent arrangement that allows
light transmission and refraction. The corneal epithelium is the outermost layer; it is made up of
five to seven layers of nonkeratinized, stratified epithelia. The basal epithelial cells of this layer
have a prominent nucleus and are mitotically active, as epithelial cells turn over every five to
seven days. The basal cells adhere to the basement membrane via a complex that also anchors
the epithelium to Bowmans layer. Subjacent to the epithelium lies the stroma, which is the
thickest layer, accounting for approximately 90% of the cornea. It is composed of highly
organized connective tissue (predominantly collagen type I fibrils) arranged in orderly sheets that
form lamellae, admixed with proteoglycans that maintain the regular spacing between the
lamellae. A meshwork of flat cells (called keratocytes) is also found between the collagenous
lamellae. Keratocytes slowly secrete collagen and ECM components that are needed to maintain
the stroma. The endothelium is the innermost layer of the cornea, formed by a single layer of
polygonal cells which secrete Descemets membrane and regulate water content in the stroma via
Na+/K+-ATPase pumps. Descemets membrane is composed mostly of collagen type IV,
glycoproteins and fibrin. Dysfunction of the endothelium causes stromal edema and corneal
opacity. In addition to these components, the cornea also contains numerous nerve endings (300-
400 times more than similarly-sized sections of skin). Most of these nerves are sensory, derived
from the ciliary nerves of the trigeminal ganglion ophthalmic branch.(2)
Once the cornea is damaged, the outcome depends on the degree of injury. If only the outer
epithelium is damaged, adjacent epithelial cells will quickly migrate to cover the injury. This
initial response is immediately followed by a proliferation to regain normal epithelial thickness.
Concurrently, upon receiving signals from the damaged epithelium (via cytokines,
neuropeptides, growth factors, lipid mediators and chemokines) underlying keratocytes undergo
apoptosis and activate adjacent keratocytes. If the stroma is damaged as well, there is a stronger
activation of keratocytes, resulting in their transformation into fibroblasts and myofibroblasts,
leading to scar tissue formation and loss of transparency.(2)
Corneal injury will also result in an inflammatory response, generally characterized by
infiltration of the stroma by neutrophils and proinflammatory lipid mediators. If this
inflammation is not resolved, then corneal fibrosis, pigmentation, and neovascularization occur,
ultimately resulting in scarring and disruption of the blood-ocular barrier.(2)
References:
1. Blood DC, Studdert V. Bailli+�-�re's comprehensive veterinary dictionary. London, UK: 1, Balli+�-�re Tindall; 1990.
2. Kenchegowda S, Bazan HEP. Significance of lipid mediators in corneal injury and repair.
J Lipid Res. 2010;51(5):879-891.
3. Wilcock BP. Eye and ear. In: Maxie MG, ed.Â
Jubb, Kenndey, and Palmer's pathology of domestic animals. 5th ed. Vol. 1. Philadelphia, PA: Elsevier Saunders; 2007:459-552.