Signalment:
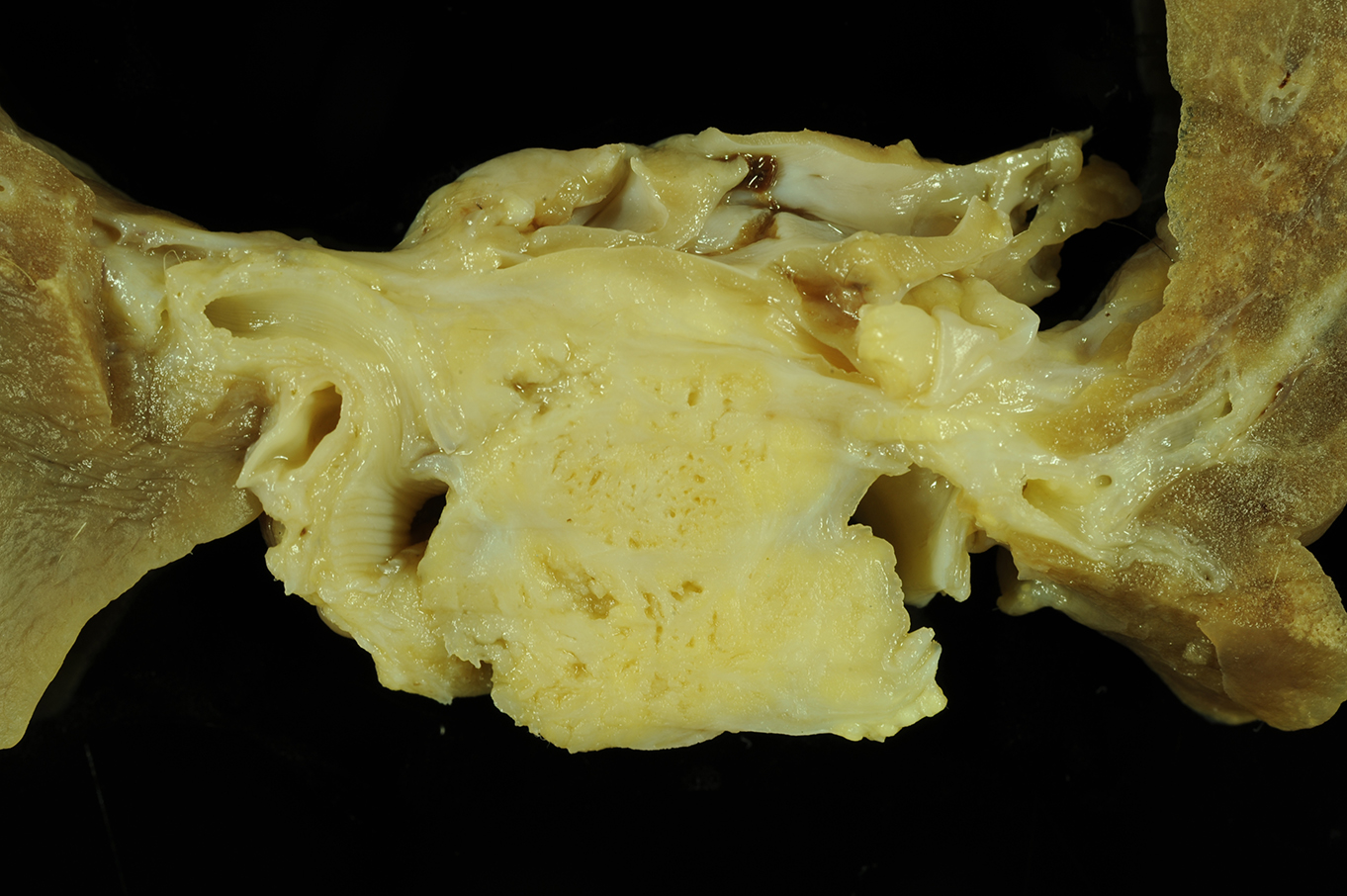
Gross Description:
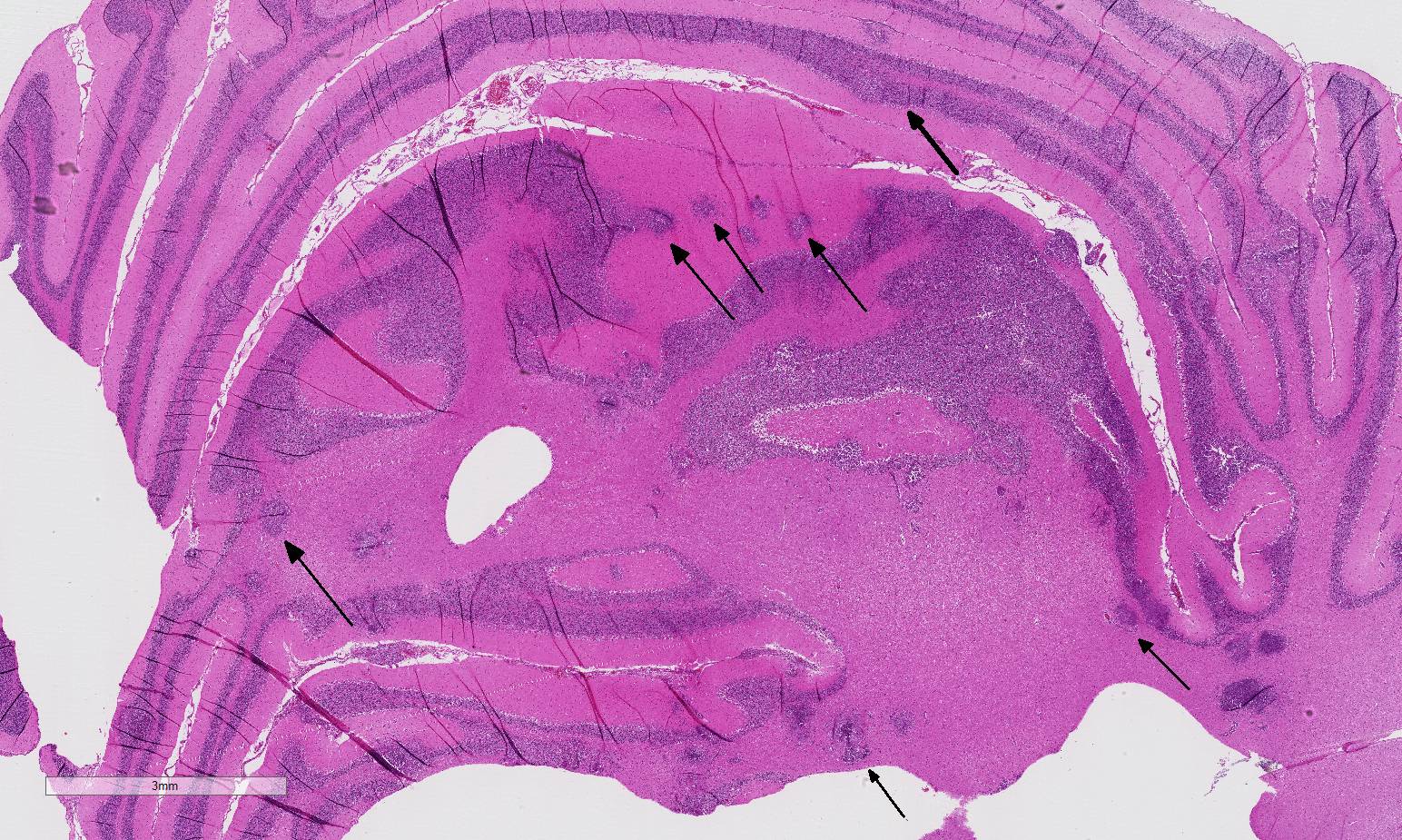
Histopathologic Description:
Morphologic Diagnosis:
Cerebellum and brain stem:
1. Encephalitis, pyogranulomatous to necrotizing, severe, multifocal, chronic with microgliosis; 2. Periventricular astrocytosis with intranuclear and/or cytoplasmic, viral inclusion bodies;
3. Meningitis, lymphocytic, mild, multifocal, chronic.
Lab Results:
Condition:
Contributor Comment:
Immunohistochemistry for CDV antigen was positive in epithelial cells of the lung, affected abdominal skin, and in periventricular astrocytes of the brain stem. Canine distemper virus is a morbilliviral disease of the family Paramyxoviridae, including measles virus and rinderpest virus. The host spectrum comprises various species of Canidae, Mustelidae, Pro-cyonidae, Phocidae, Felidae and other. The virus represents an important infectious disease in many parts of the world. CDV is usually transmitted through inhaled aerosols or close contact. First steps of the pathogenesis include infection of mac-rophages of the upper respiratory tract or the lung, which migrate to local lymph nodes and tonsils. Afterwards the virus replicates in local lymphoid tissue and spreads throughout the body within 2-5 days after exposure. Manifestations include bro-nchointerstitial pneumonia, demyelinating disease of the central nervous system, thymus atrophy, ocular disease including conjunctivitis, keratitis, retinitis and optic neuritis, pustular and/or hyperkeratotic cutaneous lesions, dental defects, bone lesions, and abortions.4,18 Furthermore, CDV infection leads to profound inhibition of cellular and humoral immune functions resulting in immunosuppression, lym-phocyte loss and leukopenia, what boosts susceptibility for opportunistic infections.6 Common secondary infections following CDV infection include Bordetella sp., adenovirus and Pneumocystis sp. infections of the lung as well as toxoplasmosis, Tyzzer`s disease, sarcocystosis and encephalitozoonosis. Secondary enteric infections with Cryptosporidium or attaching-and-effacing Escherichia coli are also well known.18 In this case, a secondary mixed bacterial infection with involvement of Nocardia veterana led to the pyo-granulomatous and necrotizing men-ingoencephalomyelitis. However, a primary bacterial infection followed by canine distemper cannot be excluded, but is even more unlikely because cases of Nocardia veterana described so far in man almost all occurred in severely immunocompromised patients.2 The detection of coagulase-negative staphylococci and Escherichia coli from lung tissue results most likely from secondary infection with ubiquitously existing bacteria.
Nocardia veterana has been described in 2001 for the first time when it was isolated from bronchial lavage of a human patient with a history of tuberculosis in a veterans hospital in Australia.15 Nocardia spp. are gram-positive, nonmotile aerobic actinomycetes, which are ubiquitous in the environment and cause a variety of suppurative and granulomatous infections, ranging from cutaneous mycetomas to disseminated systemic diseases.5 The majority of infections are caused by members of the Nocardia asteroides co-mplex, which includes Nocardia ast-eroides sensu strictu, Nocardia abscessus, Nocardia cyriacigeorgica and two clusters closely related to Nocardia carnea and Nocardia flavorosea.20
Organisms of the Nocardia asteroides complex are capable to cause pulmonary, systemic, central nervous and localized cutaneous nocardiosis in man and animals. Infections of bone, eyes, heart, joints and kidneys have also been reported in man as well as mammary gland infections of cows.5
In contrast to Nocardia asteroides, infections caused by Nocardia veterana are rare and have previously only been reported in man and cows with mastitis in Brazil.10 In man, Nocardia veterana infection is a rare event that is mostly promoted by preliminary immunosuppression.14 Reported cases include pneumonia in a HIV-infected patient, nodular lymphangitis in a man who was in remission from non-Hodgkin`s lymphoma, and a brain abscess in a patient with type 2 diabetes.3, 13, 17 Furthermore, Nocardia veterana infection had caused cutaneous mycetoma in a woman with systemic lupus erythematosus.16 Single cases of ascitic fluid infection and bloodstream infection in immunocom-promised man have also been described in the literature.2,10 In cows, Nocardia in-fection of the mammary gland induces severe suppurative pyogranulomatous mastitis.10 Nocardia veterana shows a high rate of multi-resistance to commonly used antibiotic drugs resulting in a failure of conventional antimicrobial agents.2,10 It has been shown that high rates of resistance for commonly used drugs in many other Nocardia spp. isolated from man exist.21
To our knowledge, this is the first report of a systemic bacterial infection associated with Nocardia veterana in a dog. These findings emphasize the risk of nocardiosis caused by Nocardia veterana in immunocompromised companion animals and a possible tra-nsmission from companion animals to immunocompromised man must be con-sidered. Furthermore, sequencing of the 16S rRNA is a suitable tool for defining different Nocardia species.9,20
JPC Diagnosis:
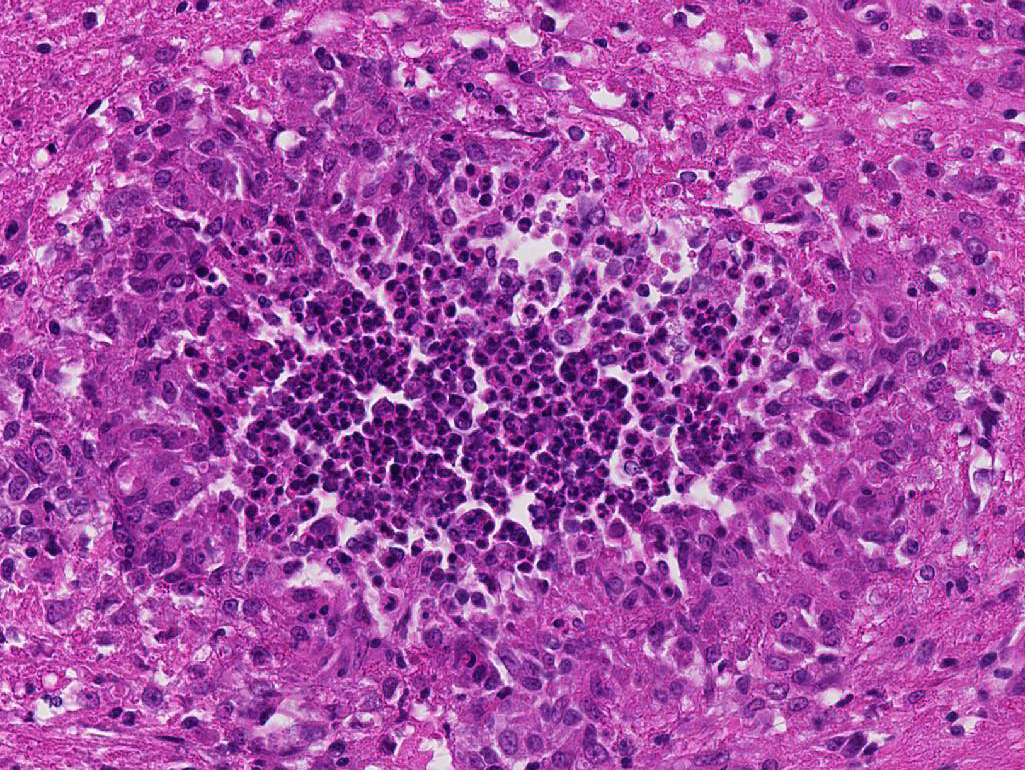
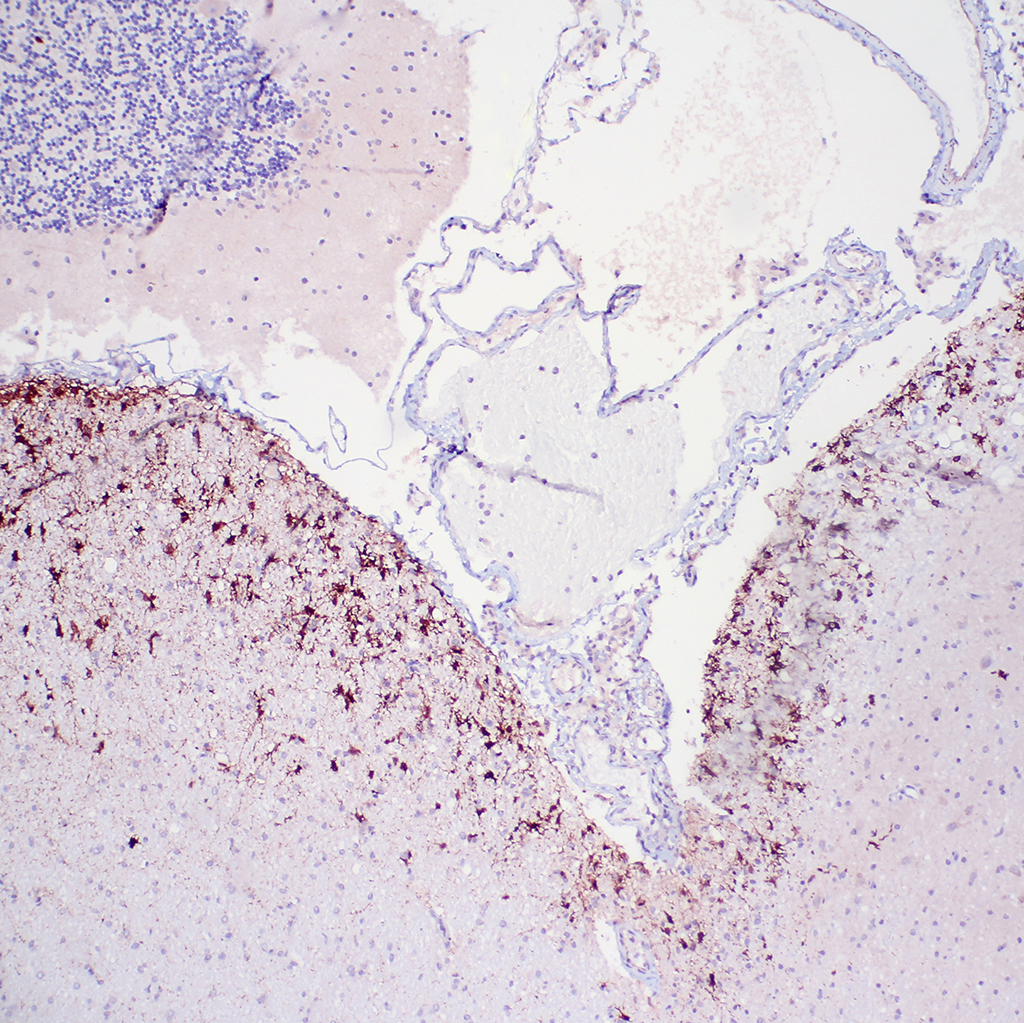
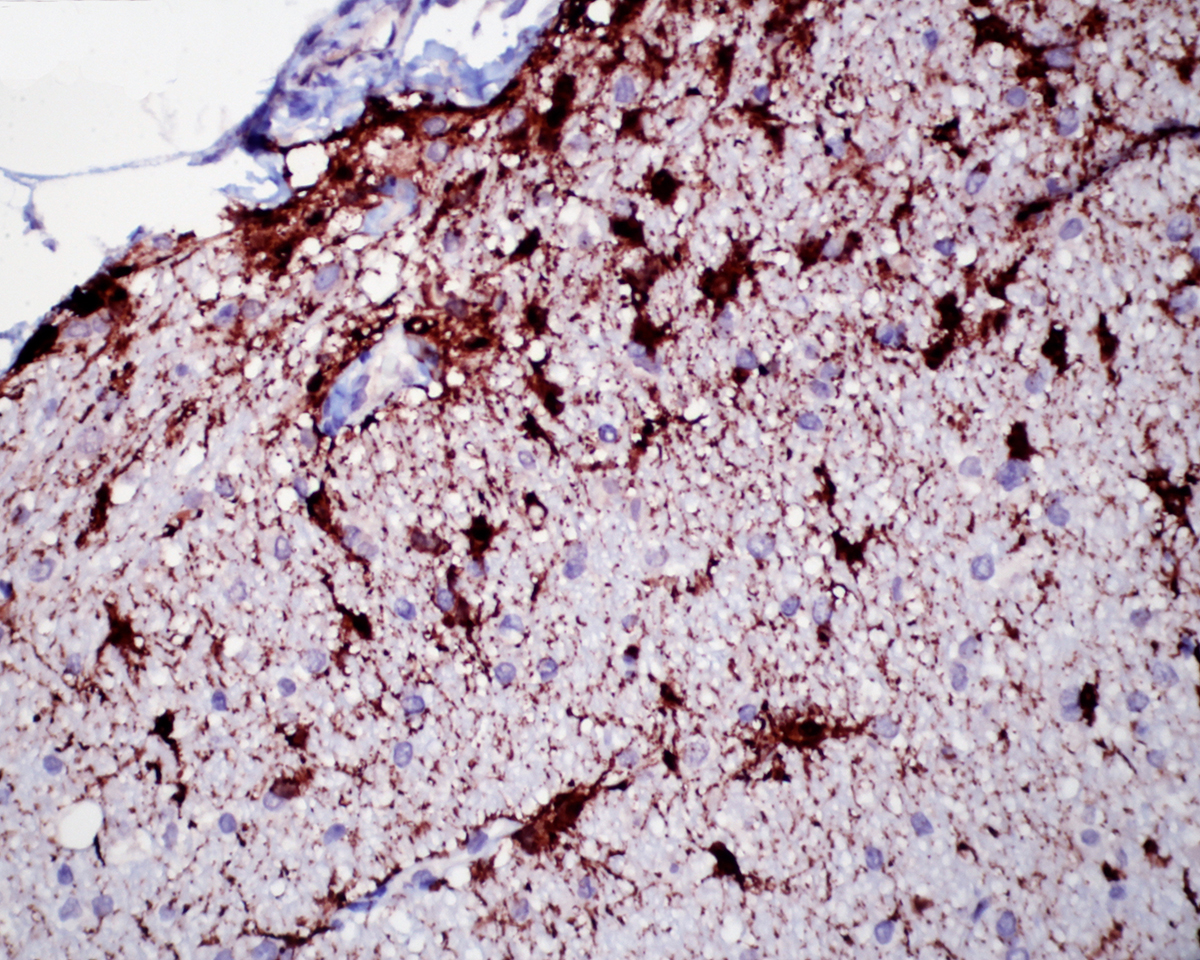
1. Cerebellum: Meningoencephalitis, pyogranulomatous, multifocal, moderate with fibrinoid vasculitis.
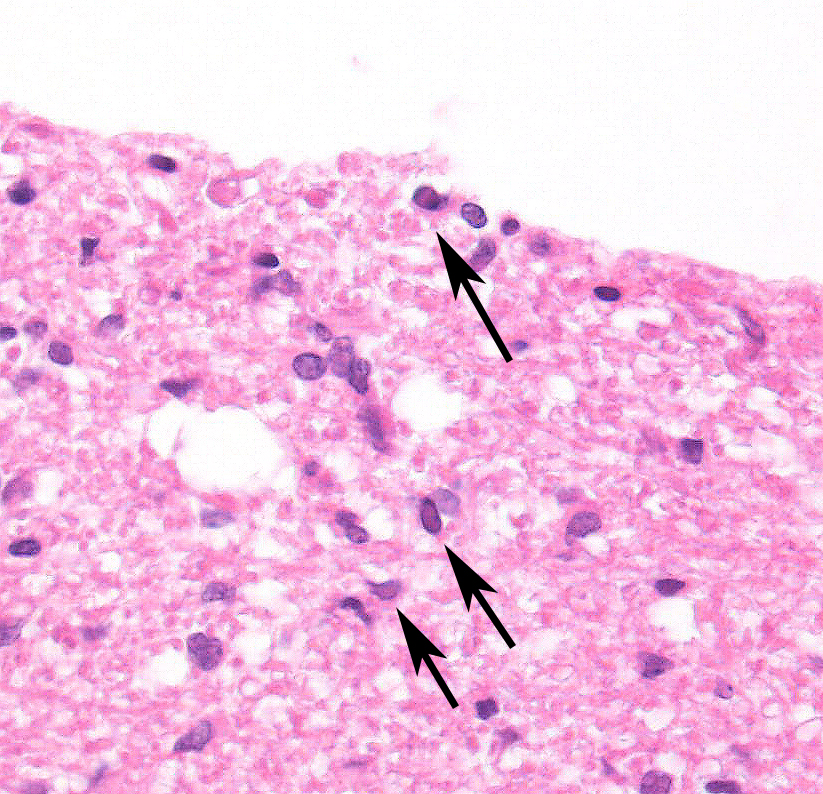
2. Cerebellum, periventricular astrocytes: Intranuclear viral inclusion bodies, numerous.
Conference Comment:
Various neurologic conditions, that are distinctive based on lesion localization and age of onset, may be associated with canine distemper virus (CDV). When the virus infects older dogs, ranging in age from 4-8 years, it can result in a chronic progressive disease referred to as multifocal distemper encephalomyelitis. Histologic lesions include a demyelinating leukoencepha-lomyelitis in the cerebellum and spinal cord.7 Old dog encephalitis is separate and rare condition that can slowly progress over a period of 3-4 months and is thought to be due to chronic, subclinical, non-replicating (persistent) infection with CDV. In this condition lesions are localized to the cerebral cortex, thalamus and midbrain and consist of a necrotizing, non-suppurative encephalitis with prominent perivascular cuffing by lymphocytes in both the grey and white matter. There is demyelination and atrophy of the cerebral white matter, and in some cases intranuclear and intracyto-plasmic inclusion bodies may be found in astrocytes. Neuronal changes such as chromatolysis and swelling may be seen in some locations.7 Typical lesions of the CNS in classic CDV infection include white matter demyelination which is prominent in the cerebellum and su-rrounding the fourth ventricle. Lesions are multifocal and there is vacuolation of the neuropil along with myelin loss, and inflammatory infiltrates are rare. Astrocytes often contain intranuclear inclusion bodies, which may be present prior to encephalomyelitis and often persist in the CNS longer than other tissues. Grey matter lesions occur less commonly and may include neuronal necrosis and mononuclear cell infiltration/nonsuppurative encephali-tis; inclusion bodies may be identified within neurons of the grey matter when affected.8
The conference histologic description was similar to the contributors description above. The salient features of the section include multifocal pyogranulomas, fibrinoid vasculitis and the presence of intranuclear viral inclusion bodies in periventricular astrocytes. Additionally, the meninges are multifocally expanded by lymphocytes, plasma cells and macrophages and there is mild multifocal expansion of Virchow-Robin space by mononuclear inflammatory cells. There is slide variation with not all slides containing choroid as well as variability in the presence and severity of fibrinoid vasculitis. Not all conference participants identified viral inclusion bodies in periventricular astrocytes, and in general, the distemper lesions in these sections were extremely subtle. Inclusions were most predictable in the periventricular cells, and were demonstrated in low numbers during the conference. Various differential dia-gnoses for pyogranulomatous men-ingoencephalitis were discussed including systemic mycotic infections such as blastomycosis and crytococcosis. Other considerations include protothecosis and mycobacteriosis.7 Silver and acid-fast stains repeated at JPC on the section failed to identify microrganisms. Regardless of the cause for the granulomas in this particular case, pathologists should be concerned about immunosuppression as a predisposing factor in the dissemination of potentially any secondary pathogen. The true nature of dual infections (opportunistic vs. pol-ymicrobial) has not been elucidated in most cases.
References:
1. Alves L, Khosravi M, Avila M, Ader-Ebert N, et al. SLAM- and nectin-4 independent noncytolytic spread of canine distemper virus in astrocytes. J Virol. 2015; 89:5724-5733.
2. Ansari SR, Safdar A, Han XY, O´Brien S. Nocardia veterana bloodstream infection in a patient with cancer and a summary of reported cases. Int J Infect Dis. 2006;10:483-486.
3. Arends JE, Stemerding AM, Vorst SP, de Neeling AJ, Weersink AJ. First report of a brain abscess caused by Nocardia veterana. J Clin Microbiol. 2011; 49:4364-4365.
4. Baumgärtner W, Boyce RW, Alldinger S, et al. Metaphyseal bone lesions in young dogs with systemic canine distemper virus infection. Vet Microbiol. 1995; 44:201-209.
5. Beaman BL, Beaman L. Nocardia species: host-parasite relationship. Clin Microbiol Rev. 1994;7:213-264.
6. Beineke A, Puff C, Seehusen F, Baumgärtner W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet Immunol. Immunopathol. 2009; 127:1-18.
7. Cantile C, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 2. St. Louis, MO: Elsevier; 2016:362, 384-385.
8. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 2. St. Louis, MO: Elsevier; 2016:575-576.
9. Chun J, Goodfellow M. A phylogenetic analysis of the genus Nocardia with 16S rRNA gene sequences. Int J Syst Bacteriol. 1995;45:240-245.
10. Condas LA, Ribeiro MG, Yazawa K, et al. Molecular identification and antimicrobial susceptibility of Nocardia spp. isolated from bovine mastitis in Brazil. Vet Microbiol. 2013; 167:708-712.
11. de Vries, de Swart RL. Measles immune suppression: Functional impairment or a numbers game? PLoS Pathog. 2014; 10(12):e1004482.
12. de Vries, McQuaid S, van Amerongen G, Yuksel S, et al. Measles immune suppression: Lessons from the macaque model. PLoS Pathog. 2012; 8(8):e1002885.
13. Dua J, Clayton R. First case report of Nocardia veterana causing nodular lymphangitis in an immunocompromised host. Australas J Dermatol. 2013;doi:10.1111/adj.12043.
14. Godreuil S, Didelot MN, Perez C, et al. Nocardia veterana isolated from ascetic fluid of a patient with human immunodeficiency virus infection. J Clin Microbiol. 2003; 41:2768-2773.
15. Gurtler V, Smith R, Mayall BC, Pötter-Reinemann G, Stackebrandt E, Kroppenstedt RM. Nocardia veterana sp. nov., isolated from human bronchial lavage. Int J Syst Evol Microbiol. 2001; 51:933-936.
16. Kashima M, Kano R, Mikami Y, et al. A successfully treated case of mycetoma due to Nocardia veterana. Br J Dermatol. 2005; 152:1349-1352.
17. Liu WL, Lai CC, Hsiao CH, et al. Bacteremic pneumonia caused by Nocardia veterana in an HIV-infected patient. Int J Infect Dis. 2011; 15:430-432.
18. Maxie MG, Youssef S. Respiratory system. In: Jubb KV, Kennedy P, Palmer N, ed. Patholgy of Domestic Animals. 5th ed. Philadelphia, USA: Saunders-Elsevier; 2007:635-638.
19. Mina MJ, Metcalf CJ, de Swart RL, Osterhaus ADME, Grenfell BT. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science. 2015; 348(6235):694-699.
20. Roth A, Andrees S, Kroppenstedt RM, Harmsen D, Mauch H. Phylogeny of the genus Nocardia based on reassessed 16S rRNA gene sequences reveals underspeciation and division of strains classified as Nocardia asteroides into three established species and two unnamed taxons. J Clin Microbiol. 2003;41: 851-856.
21. Schlaberg R, Fisher MA, Hanson KE. Susceptibility profiles of Nocardia isolates based on current taxonomy. Antimicrob Agents Chemother. 2014;58:795-800.