Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 21
April 11th, 2018
CASE I: Case 1 G9312 (JPC 4085100).
Signalment: 6-year-old, male, mouse lemur (Microcebus murinus), non-human primate.
History: Within a captive, indoor housed colony of grey mouse lemurs (Microcebus murinus), a male intact, six-years-old animal presented with acute onset of clinical symptoms including hematuria, decreased general condition, weight loss, and inappetence. General examination revealed a poor body condition and blood-smeared coat in the genital region. Injuries were not detected. Therefore, an acute hemorrhagic cystitis was suspected. Therapy consisted of parenteral application of enrofloxacin, meloxicam, fluid therapy, as well as vitamins and supplementary food as supportive care. Two days after initial presentation, the lemur was found dead.
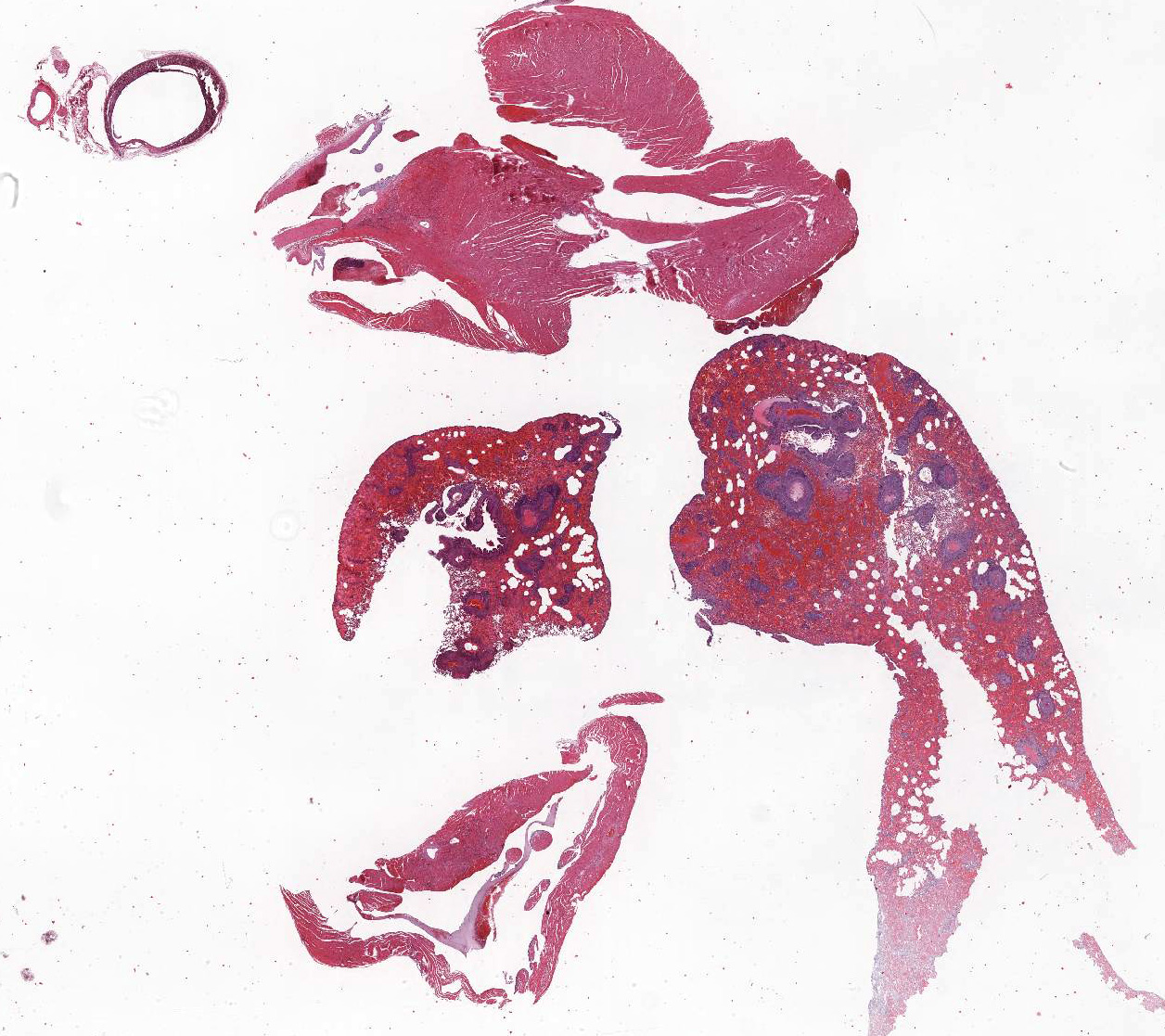
Gross Pathology: At necropsy, hemorrhages were found within different organs. Hemorrhages were most prominent within lung parenchyma and urinary bladder and less severe within renal pelvis and the subcutis. Furthermore, there was splenomegaly.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.):
Bacteriology: culture: negative
Virology: PCR for agents inducing respiratory disease (Influenza, Paramyxovirus, Human Metapneumovirus, Respiratory syncytial virus, Adenovirus): negative
Special stains used for histology: Ziehl-Neelsen stain for acid fast bacteria: negative; PAS reaction: negative; silver impregnation: negative
Microscopic Description:
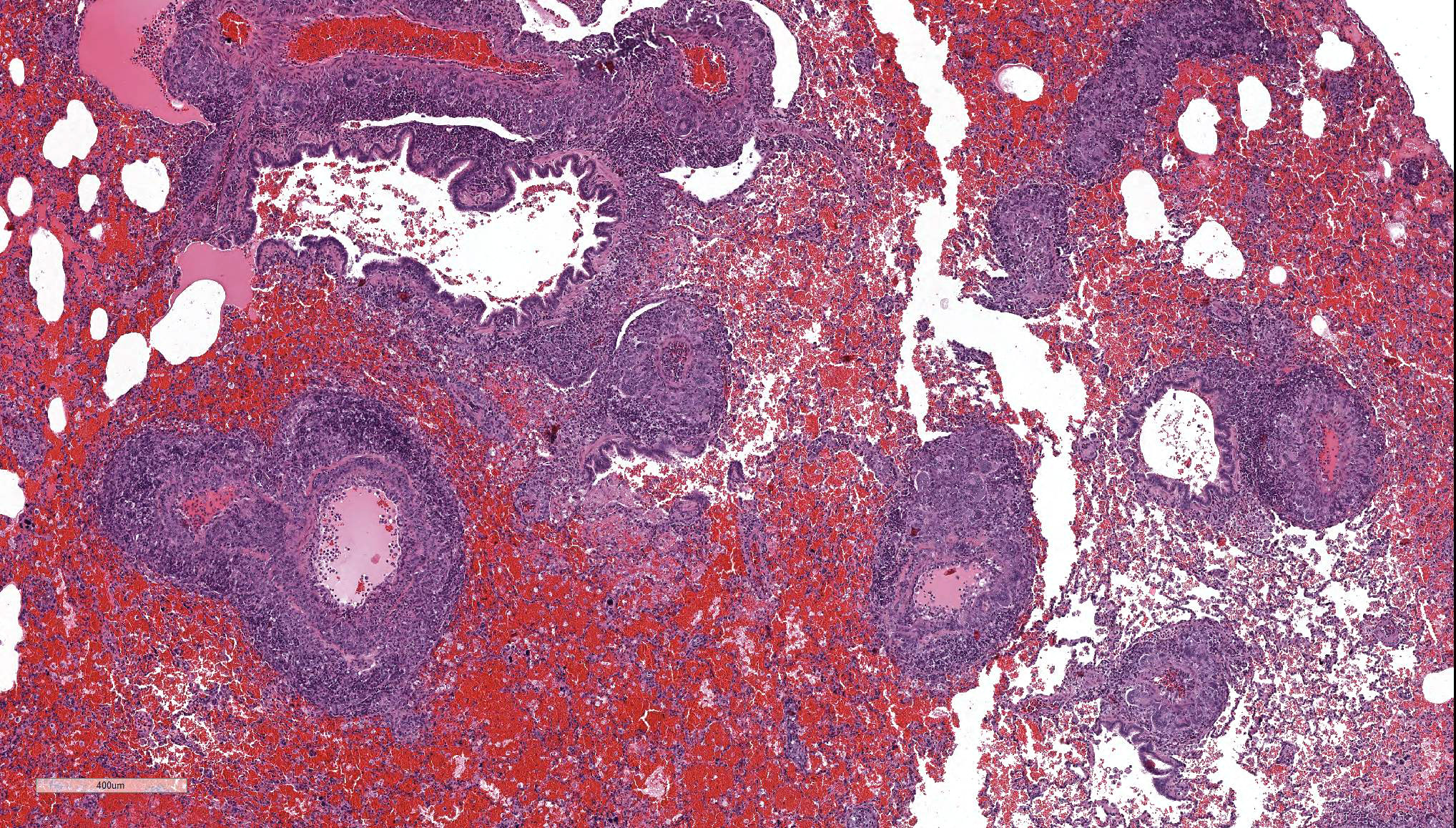
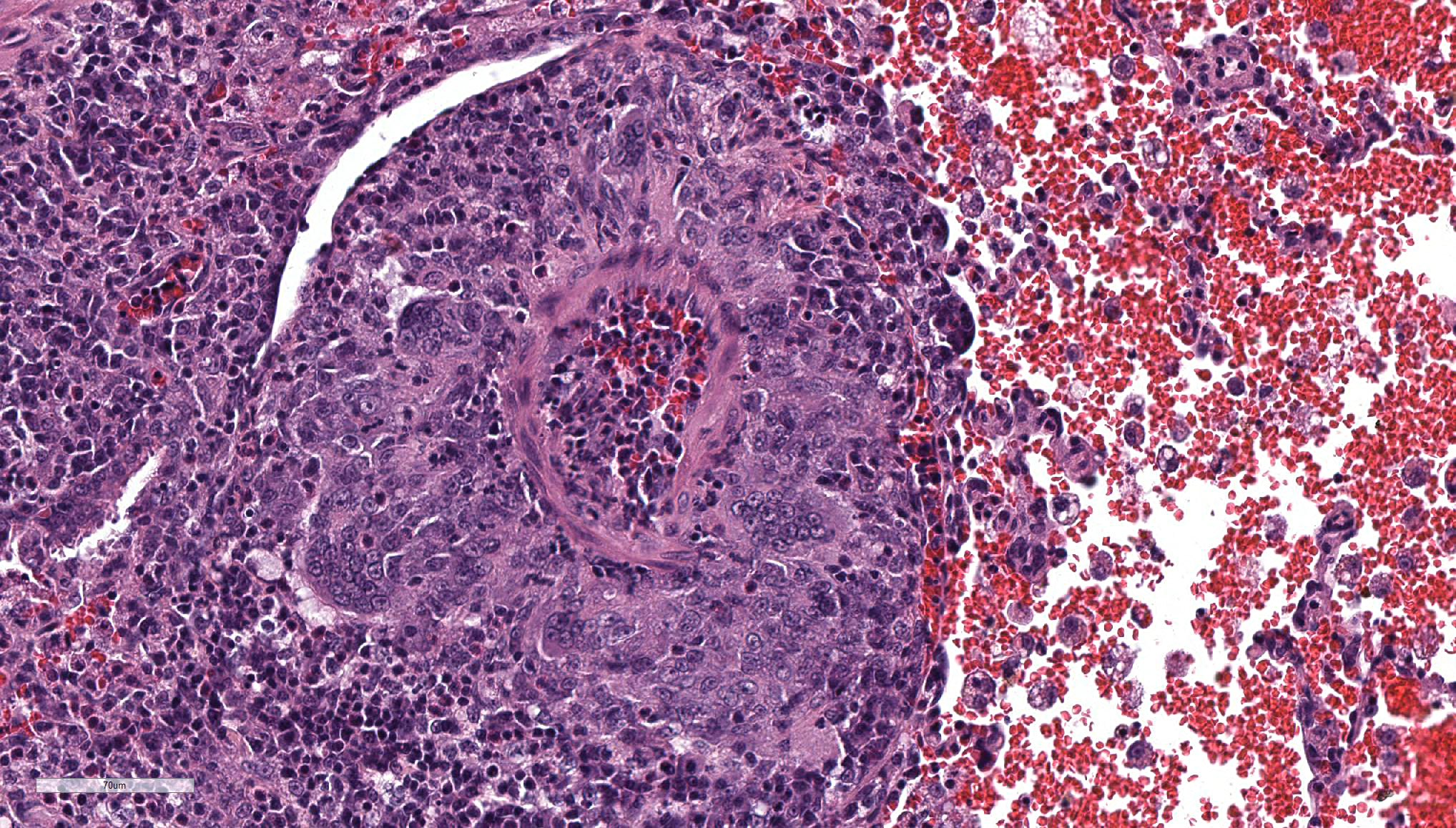
The main histologic finding within the lung parenchyma was a severe granulomatous inflammation of small- and medium-sized arteries. The intima of affected vessels showed mild fibrinoid necrosis and proliferation. The tunica media and adventitia were heavily infiltrated with a mixed cellular infiltrate. Giant cells of foreign body and Langhans’ type represent the dominant cell type. Eosinophilic cells were also present in high numbers. The lesions were accompanied by alveolar hemorrhage and histiocytosis. Comparable vascular alterations of milder degree were found within the kidneys. In the kidneys, giant cells were missing. Furthermore a mild lymphocytic interstitial myocarditis was found. Reactive extramedullary hematopoiesis was prominent within the spleen and with lesser extent within the liver.
Contributor’s Morphologic Diagnosis:
Lung: vasculitis, chronic, granulomatous and eosinophilic, multifocal, severe, with prominent giant cell formation, alveolar histiocytosis and alveolar hemorrhage, idiopathic, non-human primate.
Contributor’s Comment: A unique case of an idiopathic granulomatous generalized vasculitis in a mouse lemur is described. The cause of disease remains unclear. The most important infectious agents inducing granulomatous inflammation such as tuberculosis, leprosy, aspergillosis and leishmaniasis were ruled out histologically by special stains. No hints were found for a foreign body reaction or foreign body disease by histologic investigation. A drug induced vasculitis could be excluded because the animal did not receive drugs like propylthiouracil, methimazole, sulfasalazine, D-penicillamine, or minocycline capable to induce microscopic polyangitis. Therefore, an autoimmune or allergic disorder was suspected.
Several forms of idiopathic disseminated giant cell arteritis are recognized in humans and should be discussed as differential diagnosis for this case (Table 1). They mainly differ in their distribution, and a rough classification can be done according to the type of vessels involved. On this basis, arteritis temporalis (classic giant cell arteritis Horton) and Takayasu arteritis could be excluded in the present case because they mainly affect the aorta and other large-sized vessels. With the same argumentation polyarteritis nodosa (PAN), an idiopathic multisystemic necrotizing vasculitis, could be excluded too, because it mainly affects medium-sized vessels and rarely lung vasculature. The disease is well recognized in humans, and a PAN-like syndrome has been observed in a number of other species. In non-human primates, the disease is only described in cynomolgus monkeys (Macaca fascicularis).1,6 The two case reports describe a necrotizing arteritis affecting vessels in the kidney, small intestine, colon, heart, spleen, mesentery, urinary bladder, and pancreas. The pulmonary vasculature was not involved. The lesions were segmental in distribution and of varying severity and stage of development. A transmural mixed inflammatory cell infiltrate was present, often accompanied by fibrinoid necrosis of the tunica media.6 Main differences to the present case exist in the lack of giant cells and the amount of fibrinoid necrosis. For the given reasons, PAN was excluded as a diagnosis in the present case.
The eosinophilic nature of the lesions are indicative for another form of idiopathic vasculitis called eosinophilic granulomatosis with polyangitis or Churg-Strauss vasculitis. This is an autoimmune condition associated with asthma.3 The clinical history of the diseased animal gives no evidence for a preexisting asthmatic syndrome. The inflammatory character of Churg-Strauss vasculitis is predominantly eosinophilic, whereas giant cells are more prominent in the present case. For these reasons, Churg-Strauss vasculitis was also excluded as a diagnosis in the present case.
The described lesion shows more similarities with the entity granulomatosis with polyangitis (GPA), previously known as Wegener granulomatosis. The disease is an idiopathic vasculitis of medium and small arteries of the respiratory tract with coexisting glomerulonephritis. As in the presented case, hematuria is a frequent finding. The main histologic criteria are presence of giant cells and fibrinoid necrosis of the vessel wall. GPA is generally characterized by anti-neutrophil cytoplasmic antibodies (ANCA).8
Nevertheless, the most probable diagnosis in this case is disseminated visceral giant cell arteritis, a giant cell arteritis of extracranial arteries and arterioles. Histologic similarities are the presence of giant cells, a mixed inflammatory infiltrate with eosinophils and less extend of fibrinoid necrosis of the vessel wall.5
The pathogenesis of all vasculitides discussed above is poorly understood and most likely involves immunopathogenic mechanisms. Most speculation centers on immune complex deposition, with subsequent activation of the complement cascade, neutrophil and monocyte chemotaxis, and the release of lysosomal enzymes, oxygen-free radicals, and proinflammatory mediators. Anti-neutrophil cytoplasmic antibodies (ANCA) have been identified in patients suffering from some forms of vasculitis. The identification of ANCA antibodies may help to discriminate among the different forms. In the present case, there was no possibility for further investigations. Therefore, the final diagnosis and pathogenesis remains speculative.
Table 1: Disseminated visceral giant cell arteritis, differential diagnoses.5
|
Pathologic entity |
Principle affected vessel |
Giant cells |
Fibrinoid necrosis |
Eosinophilic infiltrates |
ANCA |
|
Arteritis temporalis (Classic giant cell arteritis Horton) |
cranial arteries occasionally large systemic arteries |
+ |
± |
± |
|
|
Takayasu arteritis |
aorta and aortic arch branches |
± |
- |
- |
|
|
Polyarteritis nodosa |
medium sized and small arteries |
± |
+++ |
+++ |
negative |
|
Eosinophilic granulomatosis with polyangitis (Churg-Strauss vasculitis )
|
extracranial small arteries and veins, perivascular tissue |
+ |
+++ |
+++ |
positive |
|
Granulomatosis with polyangiitis (Wegener granulomatosis)
|
small vessel of upper respiratory tract, lung, kidney |
+++ |
+++ |
+++ |
positive c-ANCA |
|
Disseminated visceral giant cell arteritis |
extracranial small arteries and arterioles |
+++ |
± |
- |
negative |
Legend: -: absent; ±: occasionally present; +: usually present; +++: always present;
(adapted from Lie, 1977)
JPC Diagnosis:
- Lung, small and medium caliber pulmonary arteries: Arteritis, granulomatous, segmental, severe with diffuse, severe alveolar hemorrhage and edema, mouse lemur (Microcebus murinus), non-human primate.
- Heart, aorta and coronary artery: Arteritis and periarteritis, granulomatous, segmental, moderate with multifocal aortic valvular endocarditis.
- Heart: Myocardial degeneration and necrosis, multifocal, mild.
Conference Comment: In humans, there are two common vasculitides of medium to large vessels that can cause both peripheral and coronary artery disease: Takayasu’s arteritis and giant-cell arteritis. Giant-cell arteritis typically affects older females (greater than 50 years old) and has two variants: cranial giant-cell arteritis and large-vessel giant-cell arteritis. Clinical signs for cranial giant-cell arteritis include headaches, scalp pain, jaw claudication, and loss of vision. Patients with large-vessel giant-cell arteritis generally suffer from aortic dissection or aneurysm, claudication of the limbs, myocardial ischemia, and acute aortic insufficiency.4
Ultrasound can raise suspicion of giant-cell arteritis by identifying the “halo sign” which represents diffuse edema of the vessel wall with adjacent normal vascular wall (known as “skip lesions”). Diagnosis is by temporal-artery biopsy, but this method can result in false-negatives for two reasons: (1) sampling of the “skip lesion” region or (2) the temporal artery is not involved. The latter is fairly common in large-vessel giant-cell arteritis, in which 40% of patients do not have temporal artery involvement.4
The most commonly recommended treatment for giant-cell arteritis in humans is glucocorticoids with many patients requiring long-term treatment to prevent worsening of the arteritis and eventually occlusion of large vessels.4 New research has shown the efficacy of tocilizumab (an interleukin-6 receptor alpha inhibitor) in combination with glucocorticoids in sustaining disease remission in patients and avoiding the side effects of long-term glucocorticoid use. Elevated serum levels of interleukin-6 result in increased concentration of C-reactive protein and other acute phase proteins which correlate with disease severity. Tocilizumab, an interleukin-6 receptor alpha inhibitor, allows for reduced concentrations of glucocorticoids and decreased levels of acute phase proteins.7
There is significant variability between the submitted unstained slides and the digital slide provided to conference recipients. The moderator thought the only vessels affected were small and medium-sized pulmonary arteries because on the digital slide, the aorta and coronary arteries were not involved, but they were inflamed in our stained slide. Conference participants noted the recent article written about this case, which states that the aorta was unremarkable.2 The moderator theorized that the article was written based on initial cuts from the block, and emphasized the segmental and stochastic nature of many vasculitides.
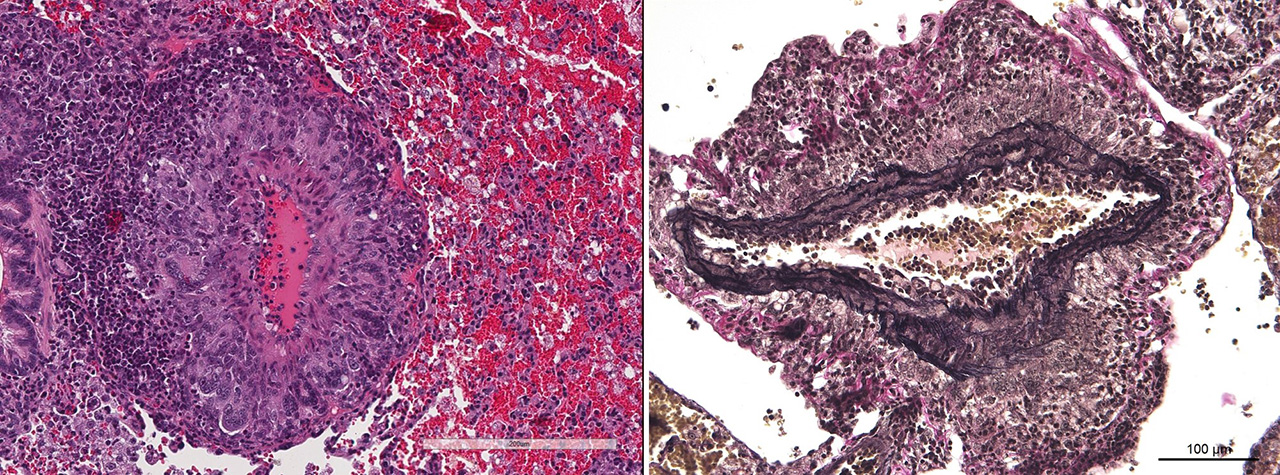
Two special stains were run, Verhoff van Giesson and Masson’s trichrome. In the elastin stain, the tunica media of the medium pulmonary arteries generally has intact elastic lamina with smooth muscle cells in between. In the Masson’s trichrome, surprisingly no fibrosis is identified within the tunica intima or media of affected arteries. In many arteries the lesions are more of a periarteritis because the tunica intima and media are largely spared. A discussion of the abundant acute hemorrhage in the alveoli included possible pulmonary hypertension leading to rupture of small alveolar capillaries. This condition is characterized by small arteries that exhibit medial hyperplasia and fibrosis, as well as pathognomonic plexiform lesions, and right ventricular hypertrophy, none of which were identifiable in this case.
In humans, the current vasculitis nosology (the Chapel Hill consensus) was revised in 20124 and is subdivided by: vessel type, characteristics of the infiltrate, and if the individual has ANCA antigen (anti-neutrophil cytoplasmic antibodies). The moderator believes the closest classification for the entity presented here is Wegener granulomatosis and Churg-Strauss vasculitis (see chart above), but that trying to pigeon hole this into a human classification may not be useful. Certain features vary slide to slide, and the amount of fibrinoid vasculitis is a minor component in the slides examined.
Contributing Institution:
German Primate Center
Kellnerweg 4, 37077
Göttingen, Germany
www.dpz.eu
References:
- Albassam MA, Lillie LE, Smith GS. Asymptomatic polyarteriitis in a cynomolgus monkey. Lab Anim Sci 1993;43: 628–629.
- Cichon N, Lampe K, Bremmer F, Becker T, Matz-Rensing K. Unique case of granulomatous arteritis in a grey mouse lemur (Microcebus murinus) – first case description. Primate Biology. 2017; 4:71-75.
- Cottin V, Cordier J-F. Churg-Strauss syndrome. Allergy 1999;4:535-551.
- Kelly NP, Gerhard-Herman M, Desai AS, Miller AL, Loscalzo J. An unusual cause of leg pain. New England Journal of Medicine. 2017; 377(23): 2267-2272.
- Lie J T. Disseminated visceral giant cell arteriitis. Histopathologic description and differentiation from other granulomatous vasculitides. Am J Clin Pathol 1978;69: 299-305.
- Porter BF, Frost P, Hubbard GB. Polyarteriitis nodosa in a cynomolgus macaque (Macaca fascicularis). Vet Pathol 2003;40:570-503.
- Stone JH, Tuckwell K, Dimonaco S, Klearman M, et al. Trial of Tocilizumab in giant-cell arteritis. New England Journal of Medicine. 2017; 377(15): 1493-1494.
- Wojciechowska J, Krajewski W, Krajewski P, Kr?cicki T. Granulomatosis with polyangiitis in otolaryngologist practice: A review of current knowledge. Clinical and Experimental Otorhinolaryngology 2016; 9: 8-13.