Signalment:
Gross Description:
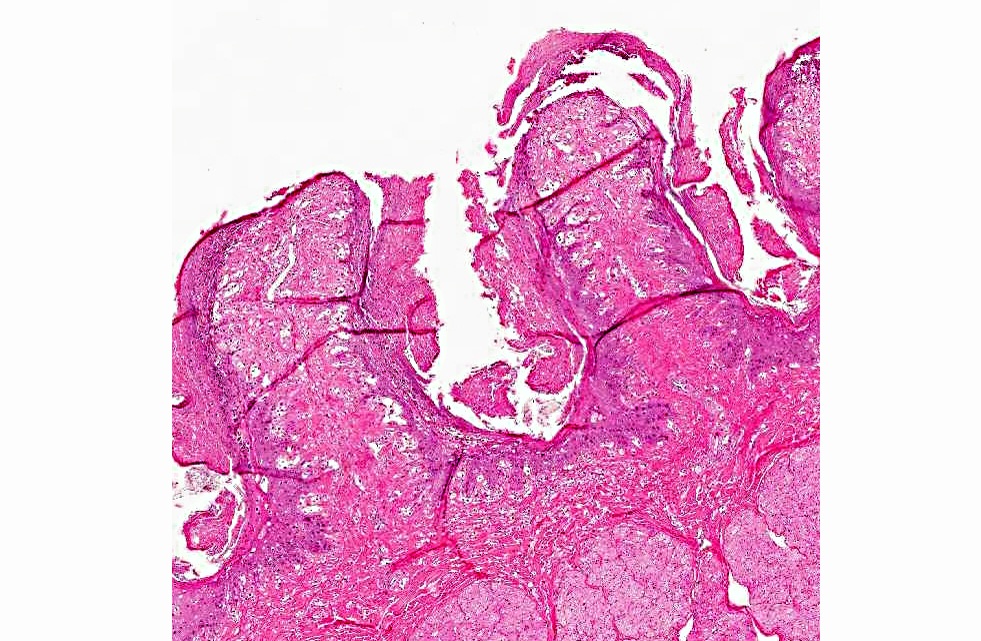
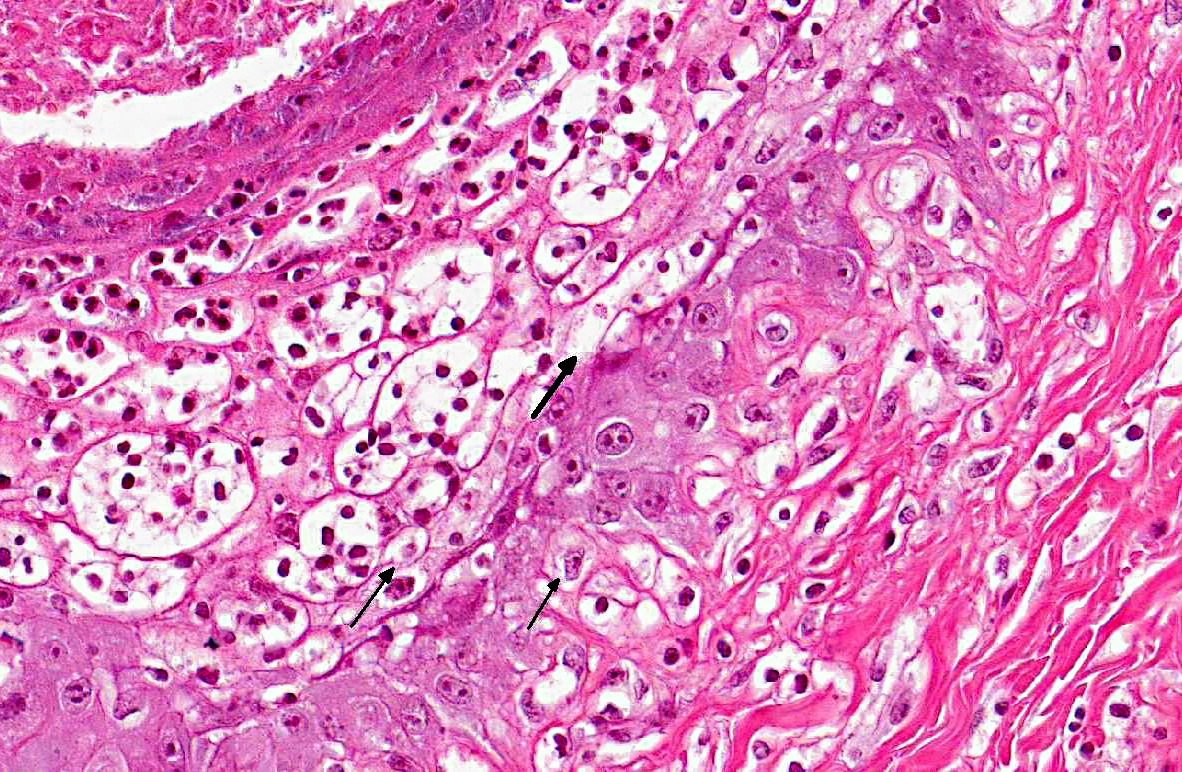
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Nearly 120 species of Baccharis have been recorded in Brazil; of those, only Baccharis coridifolia and Baccharis megapotamica have been proven to be toxic to livestock.(3,6,14,19,20) Both B. megapotamica and B. coridifolia are found in southern Brazil, but they occupy different habitats; B. megapotamica is found in marshy areas whereas B. coridifolia grows in pastureland.(3,20) Two varieties of B. megapotamica with essentially the same distribution and toxic effects on livestock are known, namely B. megapotamica var. megapotamica and B. megapotamica var. weirii.(2)0
The two varieties of B. megapotamica and Baccharis coridifolia cause a severe acute poisoning in livestock characterized by degeneration and necrosis of the epithelial lining of the gastrointestinal tract and necrosis of lymphocytes in lymph nodes, spleen, tonsils, and several lymphoid aggregates.(3,20,22) Baccharis megapotamica, B. coridifolia and B. artemisioides contain a series of potent cytotoxic agents belonging to the macrocyclic trichothecene complex of antibiotics previously believed to be produced only by fungi.(5,9) These cytotoxic substances were demonstrated to be the toxic principles in these plants.(10,22) In the case of B. megapotamica, the macrocyclic trichothecenes accumulate in the plant as baccharinoids, which are named B1, B2, B3, B4, etc. The B4 baccharinoid is most abundant, but there are also large concentrations of B1-B8. To date, no macrocyclic trichothecenes have been demonstrated in B. halimifolia, B. pteronioides, or B. glomerulifolia.Â
Spontaneous poisoning by B. coridifolia occurs frequently in cattle, occasionally in sheep and rarely in horses.(2,15,17) Spontaneous outbreaks involving B. megapotamica var. weirii have been reported in cattle, sheep and buffalo.(6,13,14) Typically, the toxicosis in livestock occurs when na+�-�ve animals raised in areas free of Baccharis spp. are transferred to pastures infested by the plant. The risk of toxicosis increases considerably if the animals are subjected to such stress factors as fatigue, hunger, or thirst; cattle that are raised in pastures where Baccharis spp. exist will graze it very rarely, if ever.(3)
Livestock will not usually ingest either variety of B. megapotamica, but a combination of hunger, dehydration, and lack of familiarity with the plant most likely led the buffalo of this report to the lethal ingestion of B. megapotamica var. weirii.(20) In the southern region of Brazil there are reports of spontaneous poisoning by four plants that induce similar disturbances in the gastrointestinal tract and should be included in the differential diagnosis, namely B. coridifolia, B. megapotamica var. weirii, Baccharidastrum triplinervium, and Eupatorium tremulum. The toxic principle of the latter two plants is as yet undetermined. Poisoning caused by the first two plants is virtually clinically and pathologically indistinguishable; however, the habitats of B. megapotamica var. weirii (marshy areas) and B. coridifolia (dry pastureland) differ, and this helps in differentiating between the two intoxications.(3,20)
Additionally, lymphoid necrosis is reportedly less severe in cases of B. coridifolia and E. tremuluman poisoning and does not occur in the poisoning caused by B. triplinervium. Lesions in the forestomachs are much less severe in B. triplinervium poisoning than in the poisonings caused by the other three plants. Larger amounts (20-30 g/kg/bw) of E. tremulum and B. triplinervium must be consumed to induce disease and death in cattle; this translates to much lower mortality ratios for these two plants when compared to Baccharis spp. toxicosis. To confirm the diagnosis of poisoning by any of these plants it is important to find evidence of plant consumption.(13) From the standpoint of the morphological aspects of the lesions in the forestomachs, B. megapotamica var. weirii poisoning closely resembles ruminal acidosis. However, ruminal acidosis usually follows the ingestion of excess carbohydrate in the form of grain or other fermentable foodstuffs, and is associated mainly with intensive beef and dairy production and not with cattle at pasture.(4) The cause of death in cases of B. megapotamica poisoning is unknown. However, since the disease is virtually identical to B. coridifolia poisoning, comparisons can be made. Baccharis-induced death is believed to be caused by dehydration and acid-base imbalance resulting from fluid loss into the ruminal compartment in a similar manner to what happens in ruminal acidosis.(15) The finding of myriads of bacteria attached to the necrotic ruminal mucosa in cases of B. coridifolia and B. megapotamica poisoning, even in animals freshly dead, suggests the possibility that bacteremia could play a role in the mechanism of death.(15)
Trichothecenes are terpenoids, which can be divided into two groups: the simple trichothecenes (e.g., deoxynivalenol [DON], diacetoxyscirpenol [DAS], and T-2 toxin) and the macrocyclic trichothecenes (e.g., baccharinoids, roridins, and verrucarins). They exhibit a wide range of biological activity, which includes dermatonecrosis, gastroenteritis, feed refusal, coagulopathy, and immunosuppression.(1,16,21) Trichothecenes are also potent phytotoxins, and the macrocyclics are particularly toxic to plants.(9,10) The T-2 toxin and DAS are highly toxic, causing necrosis of mucous membranes (mouth, pharynx, esophagus, rumen, stomach) on contact similar to lesions produced by plant-associated macrocyclic trichothecene poisoning.(11) In this regard, the lymphoid necrosis associated with baccharinoid-induced poisoning has a close morphological resemblance with the lymphoid necrosis induced by T-2 toxin, and extracts of B. megapotamica were used in treatment trials of B-cell leukemia in rats.(9) The cytotoxicity of trichothecenes is attributed to ribosomal binding and subsequent inhibition of protein synthesis in actively dividing cells of lymph nodes, spleen, bone marrow, and thymus.(16) Induction of apoptosis in these cells by the trichothecenes is likely to contribute to lesion expression.(21) Changes in cell membrane structure, with resultant lipid peroxidation due to amphophilic trichothecene molecules, inhibition of RNA and DNA synthesis, and inhibition of mitosis are additionally recognized deleterious effects of T-2 toxin on cells.(16) Although the mode of action of macrocyclic trichothecenes in B. megapotamica on subcellular levels is not completely determined, macrocyclic trichothecenes are believed to act by compromising protein synthesis by inhibiting the peptide bond formation step, and it is fair to assume that mechanisms associated with all types of trichothecene toxicoses are similar.(1) Interesting results that could shed some light on the pathogenesis of Baccharis-induced toxicosis stemmed from experimental B. pteronioides poisoning in hamsters.(18) Hamsters in the highest dosed group (200 mg) developed multiple hemorrhagic infarcts in the liver and kidney, with severe hemorrhagic enteritis and severe necrotizing vasculitis with vascular thrombosis of hepatic and renal vessels associated with fibrin thrombi in glomerular capillaries. The authors of the hamster study compared their findings to those of bacterial endotoxinproduced vasculitis and infarction.(18)
The diagnosis of B. megapotamica toxicosis in the buffalo of this report was made based on the characteristic acute clinical disease, the presence of the plant in large amounts in its characteristic habitat, and the experimental reproduction of the disease by feeding the plant present in the pasture to a calf. Baccharis megapotamica var. megapotamica and var. weirii had been previously experimentally fed to calves and lethal doses were determined to be between 3 and 4 g/kg/bw for var. megapotamica and 1 g/kg/bw for var. weirii.(20) Fatal poisoning was acute with both varieties. The most important postmortem findings were edema of the ruminal wall along with congestion of the rumen, abomasum, small intestine, cecum, and colon. Histologically, the rumen showed necrosis characterized by pyknosis and karyorrhexis of epithelial cells, mainly of the stratum spinosum. Lymphoid tissue (spleen, lymph nodes, Peyers patches) showed necrosis characterized by pyknosis and karyorrhexis of the lymphoid cells. Thus, the disease observed in the spontaneous outbreak in buffalo was essentially the same as described previously in cattle and sheep.(6,14,20)
JPC Diagnosis:
Conference Comment:
References:
2. Alda JL, Sallis ESV, Nogueira CEW et al. Intoxica+�-�+�-�o espont+�-�nea por Baccharis coridifolia (Compositae) em eq+�-+inos no Rio Grande do Sul. Pesq Vet Bras. 2009;29:409-414.
3. Barros CSL. Livestock poisoning by Baccharis coridifolia. In: Garland T, Barr AC, eds. Toxic Plants and Other Natural Toxicants Wallingford, Oxfordshire, UK: CAB International; 1998:569572.
4. Brown CC, Baker DC, Barker IK. Rumenitis and acidosis caused by carbohydrate overload. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Saunders; 2007:46-48.Â
5. Busam L, Habermehl GG. Accumulation of mycotoxins by Baccharis coridifolia: a reason for livestock poisoning. Naturwissenschaften. 1982;69:392-393.
6. Driemeier D, Cruz CEF, Loretti AP. Baccharis megapotamica var. weirii poisoning in Brazilian cattle. Vet Hum Toxicol. 2000;2:220-221.
7. Duncan WH, Piercy PL, Feurt SD, et al. Toxicological aspects of southeastern plants. II. Compositae. Econ Bot. 1957;11:7585.
8. Everist SL. Poisonous Plants of Australia. Sidney, Australia: Angus and Robertson; 1981:160-161.
9. Jarvis BJ, Midiwo JO, Bean GA, et al. The mystery of trichothecene antibiotics in Baccharis species. J Nat Prod. 1988;51:736-744.
10. Jarvis BJ, Mokhtari-Rejali N, Schenkel EP, et al. Trichothecene mycotoxins from Brazilian Baccharis species. Phytochemistry. 1991;30:789-797.
11. Jones TC, Hunt RD, King NW. Veterinary Pathology. 5th ed. Baltimore, MD: William & Wilkins;1997.
12. Marsh CD, Clawson AB, Eggleston WW. Baccharis pteronioides as a poisonous plant of the southwest. J Am Vet Med Assoc. 1920;57:430-434.
13. Oliveira-Filho JC, Carmo PMS, Lucena RB, et al. Baccharis megapotamica var. weirii poisoning in water buffalo (Bubalus bubalis). J Vet Diagn Invest. 2011;23:610-614.
14. Pedroso PMO, Bandarra PM, Feltrin C, et al. Intoxica+�-�+�-�o por Baccharis megapotamica var. weirii em ovinos. Pesq Vet Bras. 2010;30:403-405.
15. Rissi DR, Rech RR, Fighera RA, et al. Intoxica+�-�+�-�o espont+�-�nea por Baccharis coridifolia em bovinos. Pesq Vet Bras. 2005;25:111-114.Â
16. Rocha O, Ansari K, Doohan FM. Effects of trichothecene mycotoxins on eukaryotic cells: a review. Food Addit Contam. 2005;22:369-378.
17. Rozza DB, Raymundo DL, Corr+�-�a AMR, et al. Intoxica+�-�+�-�o espont+�-�nea por Baccharis coridifolia (Compositae) em ovinos. Pesq Vet Bras. 2006;26:21-25.Â
18. Stegelmeier BL, Sani Y, Pfister JA. Baccharis pteronioides toxicity in livestock and hamsters. J Vet Diagn Invest. 2009;21:208-213.Â
19. Tokarnia CH, D+�-�bereiner J. Intoxica+�-�+�-�o experimental em bovinos por mio-mio Baccharis coridifolia. Pesq Agropec Bras Ser Vet. 1975;10:79-97.
20. Tokarnia CH, Peixoto PV, Gava A, et al. Intoxica+�-�+�-�o experimental por Baccharis megapotamica var. megapotamica e var. weirii (Compositae) em bovinos Pesq Vet Bras 1992;12:19-31.
21. Uzarski RL, Islam Z, Pestka JJ. Potentiation of trichothecene-induced leukocyte cytotoxicity and apoptosis by TNF alpha and Fas activation 2. Chem Biol Interact. 2003;146:105-119.
22. Varaschin MS, Barros CSL, Jarvis BB. Intoxica+�-�+�-�o experimental por Baccharis coridifolia (Compositae) em bovinos. Pesq Vet Bras. 1998;18:69-75.