Wednesday Slide Conference, Conference 5, Case 4
Signalment:
58 month-old, female rhesus macaque (Macaca mulatta)
History:
A female rhesus macaque was infected intrarectally with simian-human immunodeficiency virus [SHIV]. The monkey was on treatment for chronic diarrhea. Twenty eight months after infection, the animal developed acute epistaxis, ataxia, and a left-sided head tilt. The monkey was humanely euthanized due to worsening neurologic deficits.
Gross Pathology:
The monkey was in lean body condition with adequate hydration. The right axillary lymph node and spleen were mildly enlarged. No lesions were present in the heart, lungs, liver, kidneys, gastrointestinal or reproductive tract.
Although epistaxis was not noted grossly, there was blood on the swab collected for bacterial culture.
Laboratory Results:
Nasal culture: Bordetella bronchiseptica
No pathogens were noted by fecal bacterial culture. PCR results were negative for Enterocytozoon bieneusi and Cryptosporidium parvum of the liver, Mycobacterium sp. of the mesenteric lymph node and Helicobacter sp. of the stomach.
Microscopic Description:
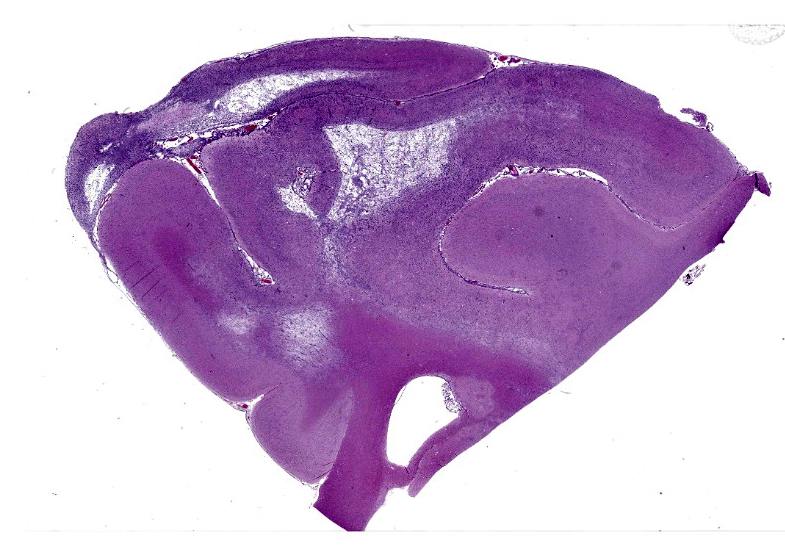
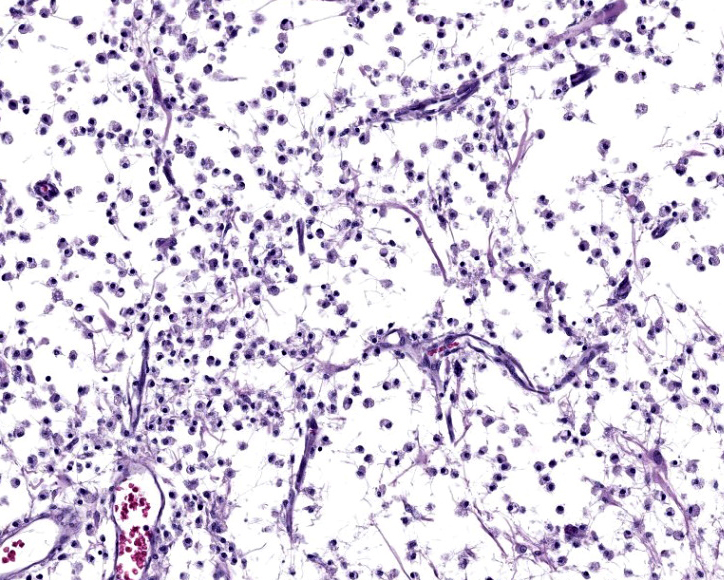
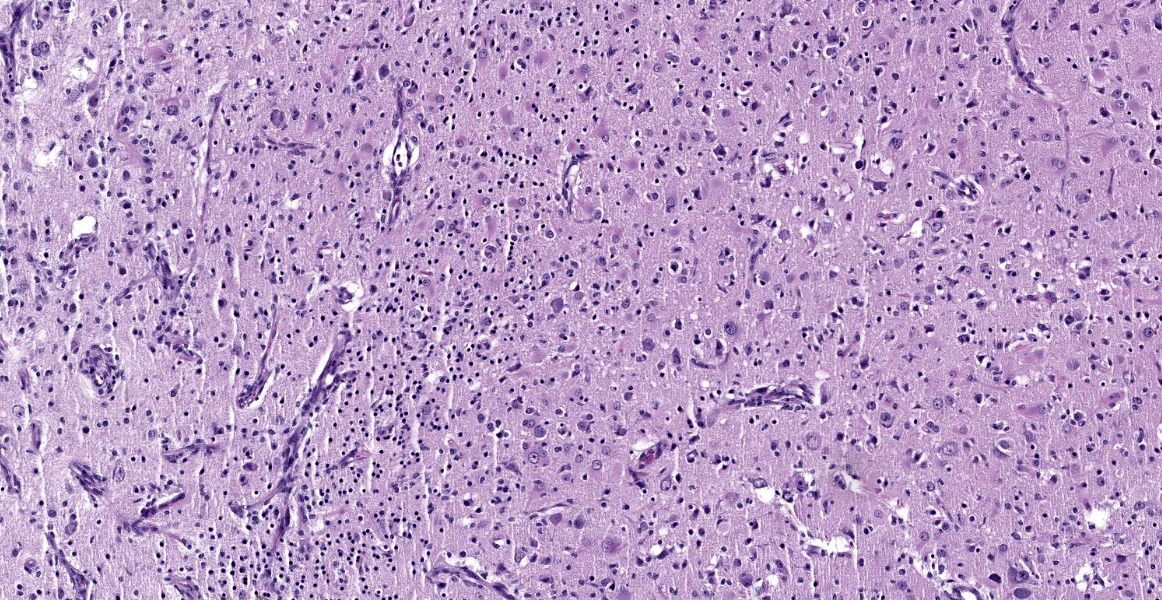
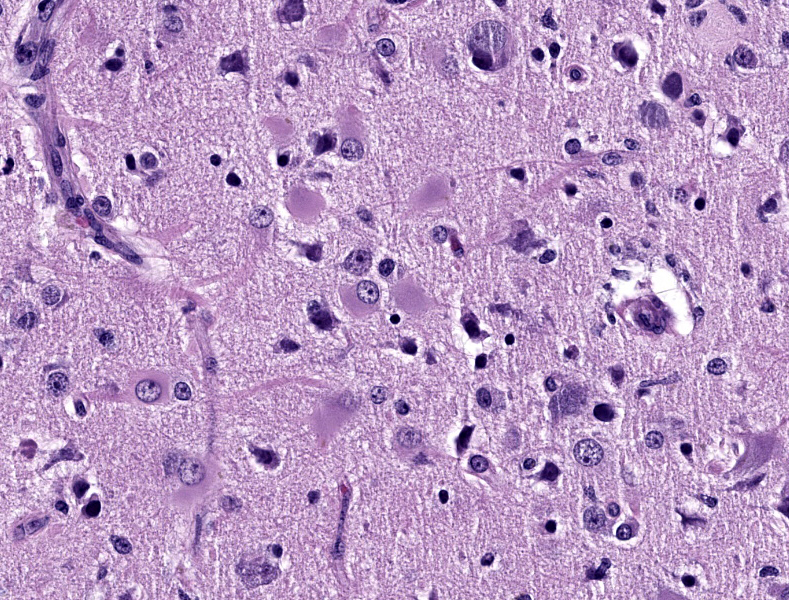
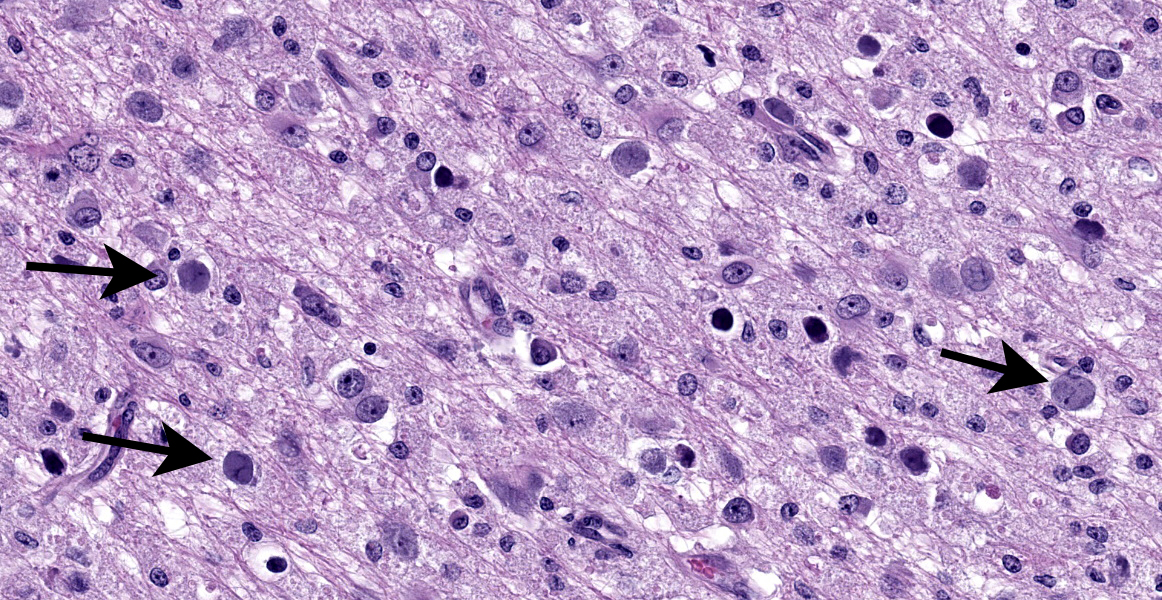
Cerebrum: Within white matter, there is multifocal, severe parenchymal loss/cavitation with numerous central Gitter cells. Around the edges of the cavities there is marked gliosis. Oligodendrocytes are markedly enlarged, up to 5x normal, and contain variably-sized, basophilic – ground glass inclusions. Reactive(gemistocytic) astrocytes are markedly pleomorphic, bizarrely shaped, bi- or trinucleated and contain large, basophilic - ground glass inclusions. Similar astrocytes are present in the superficial cortex. Numerous vessels with markedly hypertrophic endothelial cells extend from the meninges and are present around and within the areas of cavitation. Few neutrophils and lymphocytes are present around some vessels. In the meninges, few lymphocytes, plasma cells and macrophages are present.
PCR: SV40 was not detected on formalin fixed brain.
EM: Within the nucleus of oligodendrocytes, there are numerous, tightly packed, 40-45 nm viral particles arranged in paracrystalline arrays.
Contributor’s Morphologic Diagnosis:
Cerebrum: Leukoencephalomalacia, severe with oligodendrocytic and astrocytic intranuclear inclusions and marked vascular hyperplasia.
Condition: Progressive multifocal leukoencephalopathy
Contributor’s Comment:
SV40, Simian vacuolating virus 40, is a nonenveloped, 40 - 45nm, double stranded, circular, DNA virus of the polyomavirus family.6,9,11 Transcription of the early regions of the viral genome produces large tumor antigen (LTag) and small tumor antigen (STag) proteins. Transcription of the late regions produces capsid proteins VP1, VP2, and VP3 and agnoprotein.5,9,10
Viral infection begins with VP1, VP2 and VP3 receptor mediated endocytosis.9 Once in the nucleus, viral LTag stimulates host cell DNA synthesis and induces the cell to enter into S-phase and begin DNA synthesis. Small tumor antigen (STag) is not necessary for viral replication, but it enhances efficiency of viral replication by stimulating cells to move from G0/G1 to S phase.10 Agnoprotein regulates viral proliferation.5
In addition to its capacity to cause lytic infection, SV40 LTag can cause malignant transformation by binding to and inactivating tumor suppressor proteins, retinoblastoma (pRb), p53, p107 and p130.10 SV40 can cause tumors in vitro, or when the virus is injected into rodents or non-native hosts.6,10,11
SV40 is endemic in Rhesus macaques and is spread by exposure to bodily fluids such as urine or respiratory droplets. SV40 infection causes inapparent infection or mild respiratory disease or cystitis. After a period of viremia and viruria, the virus then settles in the kidneys and remains latent in immunocompetent animals.6,11 In SIV (+) animals, virus may be found in kidney, brain, lungs and mononuclear cells.6 Clinical disease occurs after immunosuppression due to SIV/SHIV infection, treatments with immunomodulatory medications or antirejection drugs given after experimental organ transplantation.11-13 Clinical signs may be nonspecific, or due to other opportunistic infections, and include anorexia, diarrhea, anemia, and tissue wasting. Neurologic signs may include head tilt, ataxia, and blindness.8 At necropsy, multifocal malacia may be visible at the junction of grey and white matter and subependymal grey matter of the cerebrum.13
In the CNS, two types of lesions may be found. The first resembles the changes seen in progressive multifocal leukoencephalopathy (PML) in humans. Demyelination due to infection and destruction of oligodendrocytes are found multifocally in white matter, especially at the junction with gray matter. Changes also may be found in subependymal grey matter.6,11 Around foci of demyelination, remaining oligodendrocytes are enlarged and may contain basophilic – ground glass intranuclear inclusions. Gemistocytic astrocytes have a bizarre appearance and also may have inclusions. Numerous Gitter cells contain myelin debris.6,11,13 The second type of lesion is meningoencephalitis of the superficial grey matter with little demyelination. In this form, inflammation expands the meninges and extends along vessels into the grey matter. More astrocytes than oligodendrocytes are affected although both cell types can have intranuclear inclusions. Vessels in affected areas have hypertrophic endothelial cells.11,13 Both PML lesions and meningoencephalitis may be present in the same animal.11
In addition to the microscopic lesions in the CNS, there may be changes in the kidneys and lungs. In the kidneys, often at the corticomedullary junction, enlarged and vacuolated tubular or necrotic epithelial cells containing intranuclear inclusions along with tubulointerstitial nephritis may be present. In the lungs, there may be interstitial pneumonia with intranuclear inclusions in hypertrophic type 2 pneumocytes.6,11-13 On electron microscopy, 45nm particles are arranged in paracrystalline arrays within the nucleus.6
Macaques, African green monkeys, baboons, chimpanzees, marmosets and squirrel monkeys, can be infected by polyoma virus.11,13 Polyomaviruses in other species include:
Raccoons: Racoon polyomavirus: malignant peripheral nerve sheath tumors and olfactory sarcomas.4
Cattle: BoPy-V1, or Epsilon Polyomavirus bovis: severe tubulointerstitial nephritis with tubular epithelial cell necrosis has been reported in a fetus. However, infection in older cattle and humans has not been found to cause clinical disease.5
Budgerigars: Avian polyomavirus: Budgerigar fledgling disease, presents with hepatitis, ascites, and hydropericardium.7
Geese: Goose hemorrhagic polyoma virus: Hemorrhagic nephritis and enteritis disease, goslings die acutely with hemorrhagic nephritis and subcutaneous edema.7
In man, BK and JC polyomaviruses are widely spread and have a 70%-75% homology with SV40.9,11 Most people are infected with BK and/or JC viruses in childhood and 70-90% of people are seropositive by the time they reach adulthood.6,9,11,12 Reactivation of either virus by HIV infection, treatment with immunomodulatory drugs or immunosuppression following kidney/organ/bone marrow transplantation can lead to CNS or renal disease.6,9 Reactivation of BK polyomavirus in kidney transplant recipients leads to nephropathy/nephritis, hemorrhagic cystitis and urethral stenosis and may lead to graft failure.9,11,12 Reactivation of JC polyomavirus leads to progressive multifocal leukoencephalopathy characterized by demyelination with intranuclear inclusions in the oligodendrocytes. Effective treatment of HIV by antiviral medications has led to a drop of PML seen in individuals with AIDS.9
Laboratory diagnosis, except for urine cytology, is based on detecting SV40 LTag, to include via PCR in CSF, urine, or sera.3,9 Immunohistochemistry or in situ hybridization using an anti-SV40 LTag antibody can detect polyoma virus in renal or brain biopsies.9 In urine samples, decoy cells, epithelial cells with intranuclear inclusions, and cytoplasmic fragmentation, may be used as a screening tool but inclusions due to CMV and adenovirus may look similar.3 Progression of PML can be monitored by MRI.1
Polio vaccines that had been grown on rhesus kidney cell cultures and given to people from 1955 to 1963 were found to have been contaminated by SV40.11 Due to the capacity of SV40 virus to induce malignant transformation in cell culture and cause tumors in rodents, there has been controversy over whether there has been an increased rate of cancer in people who received the contaminated vaccine. Studies have had conflicting results due to different methodologies, cross reactivity with BK and JC viruses and common seropositivity for SV40.10,11
Twelve new human polyoma viruses have been identified since 2007. Of the 12 new viruses, integrated BK virus has been found rarely in urological cancers. In addition, 80% of Merkel cell carcinomas, an aggressive form of skin cancer, have integrated Merkel cell polyomavirus. It may be that with newer, more sophisticated gene sequencing techniques, the association of polyomavirus and cancer may become clearer.10
Contributing Institution:
Matthew Starost, DVM, PhD.
National Institutes of Health
9000 Rockville Pike
Building 28A
Room 111
Bethesda, MD 20892
starostm@ors.od.nih.gov
JPC Diagnosis:
Cerebrum: Leukoencephalomalacia, multifocal to coalescing, with marked gliosis, gemistocytic astrocytosis, and glial intranuclear viral inclusions.
Cerebrum: Meningioangiomatosis, focally extensive, severe.
JPC Comment:
The contributor provides a detailed summary of SV40 that accompanies an equally impressive slide submission for this final case. We have covered SV40 leukoencephalomalacia in a rhesus macaque before (Conference 13, Case 3, 2015-2016) though this case presents outstanding histologic changes. The degree of loss of white matter (cavitation), the vascular changes, and the increased cellularity (basophilia) of the neuropil make this case almost diagnostic at subgross magnification (Figure 4-1). As the contributor notes, both oligodendrocytes and astrocytes contain characteristic intranuclear inclusions (Figure 4-5) that explain the pathogenesis of this lesion. A Luxol fast blue and modified Bielschowsky silver stain highlighted the severe loss of myelinated axons in this case. Though not particularly required at this point in lesion development, these stains may be helpful for less dramatic cases.
Conference participants were intrigued about the profound vascular changes within the cortical gray matter overlying the cavitated areas. We consulted with Dr. Andrew Miller (Cornell University) about this atypical meningovascular proliferation and ultimately decided to add a second morphologic diagnosis for this case. We excerpt his excellent commentary in part below:
“…There are 3 primary considerations for the vascular changes noted in this case. Foremost, the vasoproliferative lesion emanates from the meninges and invades into the neuroparenchyma around penetrating blood vessels and there are clearly two different cell populations present. There is one that is more spindled and likely derived from meningothelial cells and the other is blood vessels lined by reactive endothelial cells. This pattern is typical for meningioangiomatosis, though it is unlikely to be directly related to SV40 infection but instead a consequence of the profound necrosis of the underlying neuroparenchyma.
Additionally, the animal in this case was SHIV(+) and proliferative arteriopathy remains an uncommon concurrent lesion that can also been seen in SIV infection.2 This proliferative arteriopathy can occur in a variety of organs including kidney, lung, and meningeal blood vessels where it can be associated with CNS infarction. The pathogenesis of this retroviral-associated arteriopathy is poorly understood; however, it is an important finding in a minority of SIV- and SHIV-infected macaques. That stated, these lesions are never as large or plaque-like as the area of interest in this case and typically present with single to small groups of vessels with asymmetric medial thickening, luminal narrowing, and variable thrombosis and mononuclear inflammation.2
Lastly, vasculitis and proliferative vascular lesions can also be associated with cytomegalovirus in immunosuppressed macaques though the large, intranuclear inclusions typical of those cases were absent in this case.14 The lesions of this macaque do not fit the expected presentation of either retroviral-associated arteriopathy or cytomegalovirus-associated vasculitis.”
References:
- Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. 2021 Jan;17(1):37-51.
- Chalifoux LV, Simon MA, Pauley DR, MacKey JJ, Wyand MS, Ringler DJ. Arteriopathy in macaques infected with simian immunodeficiency virus. Lab Invest. 1992 Sep;67(3):338-49.
- Geetha V, Rao L, Monappa V, Susmitha M, Prabhu R. Decoy cells in urine cytology: A useful clue to post-transplant polyoma virus infection. J Cytol. 2012 Apr;29(2):133-4.
- Giannitti F, Higgins RJ, Pesavento PA et al. Temporal and geographic clustering of polyomavirus-associated olfactory tumors in 10 free-ranging raccoons (Procyon lotor). Vet Pathol. 2014 Jul;51(4):832-45.
- Giannitti F, da Silva Silveira C, Bullock H et al. Bovine Polyomavirus-1 (Epsilonpolyomavirus bovis): An Emerging Fetal Pathogen of Cattle That Causes Renal Lesions Resembling Polyomavirus-Associated Nephropathy of Humans. Viruses. 2022 Sep 14;14(9):2042.
- Horvath CJ, Simon MA, Bergsagel DJ et al. Simian virus 40-induced disease in rhesus monkeys with simian acquired immunodeficiency syndrome. Am J Pathol. 1992 Jun;140(6):1431-40.
- Johne R, Müller H. Polyomaviruses of birds: etiologic agents of inflammatory diseases in a tumor virus family. J Virol. 2007 Nov;81(21):11554-9.
- Lednicky JA, Arrington AS, Stewart AR et al. Natural isolates of simian virus 40 from immunocompromised monkeys display extensive genetic heterogeneity: new implications for polyomavirus disease. J Virol. 1998 May;72(5):3980-90.
- Pinto M, Pinto M, Dobson S. BK and JC virus: a review. J Infect. 2014 Jan;68 Suppl 1:S2-
- Shuda, Masahiro. In: P.Boffetta and P.Hainaut, eds. Polyomaviruses in Human Cancer. Encyclopedia of Cancer. Vol 3, 3rd ed. Elsevier; 2019:266-27.
- Simon MA. Polyomaviruses of nonhuman primates: implications for research. Comp Med. 2008 Feb;58(1):51-6.
- Song M, Mulvihill MS, Williams KD, Collins BH, Kirk AD. Fatal SV40-associated pneumonia and nephropathy following renal allotransplantation in rhesus macaque. J Med Primatol. 2018 Feb;47(1):81-84.
- Wachtman L, Mansfield K. In: C. Abee, K. Mansfield, S. Tardif, T. Morris, eds. Viral Diseases of Nonhuman Primates. Nonhuman Primates in Biomedical Research. Vol 2, 2nd ed. Academic Press; 2012: 25-33.
- Yanai T, Lackner AA, Sakai H, Masegi T, Simon MA. Systemic arteriopathy in SIV-infected rhesus macaques (Macaca mulatta). J Med Primatol. 2006 Apr;35(2):106-12.