CASE II:
Signalment:
6-week-old male turkeys (Meleagris gallopavo)
History:
Grower facility in the Midwest with flock of 21,696 six-week-old Nicholas commercial tom turkeys. Birds were sourced from a single source parent flock and the hens from which the poults originated were in their 5th week of lay. Grower had over 5-year history of No Antibiotics EverTM (NAE) however had recently switched to conventional production. The birds were started on lasalocid (coccidiostat), did well through the brooder (first 4 weeks) and adjusted well to the finisher, typically a stressful transition. The birds were vaccinated against hemorrhagic enteritis at approximately 17 days and boosted at 27 days of age. The birds became severely depressed with ruffled feathers and overt signs of discomfort. Water consumption was diminished and the birds exhibited moderate flushing (diarrhea). The grower did not report any respiratory signs. Mortalities increased from 3-5/day to 60-70 birds expiring daily. The grower was advised to revaccinate for hemorrhagic enteritis and start amprolium. The consulting poultry veterinarian was summoned to the site and necropsied multiple birds.
Gross Pathology:
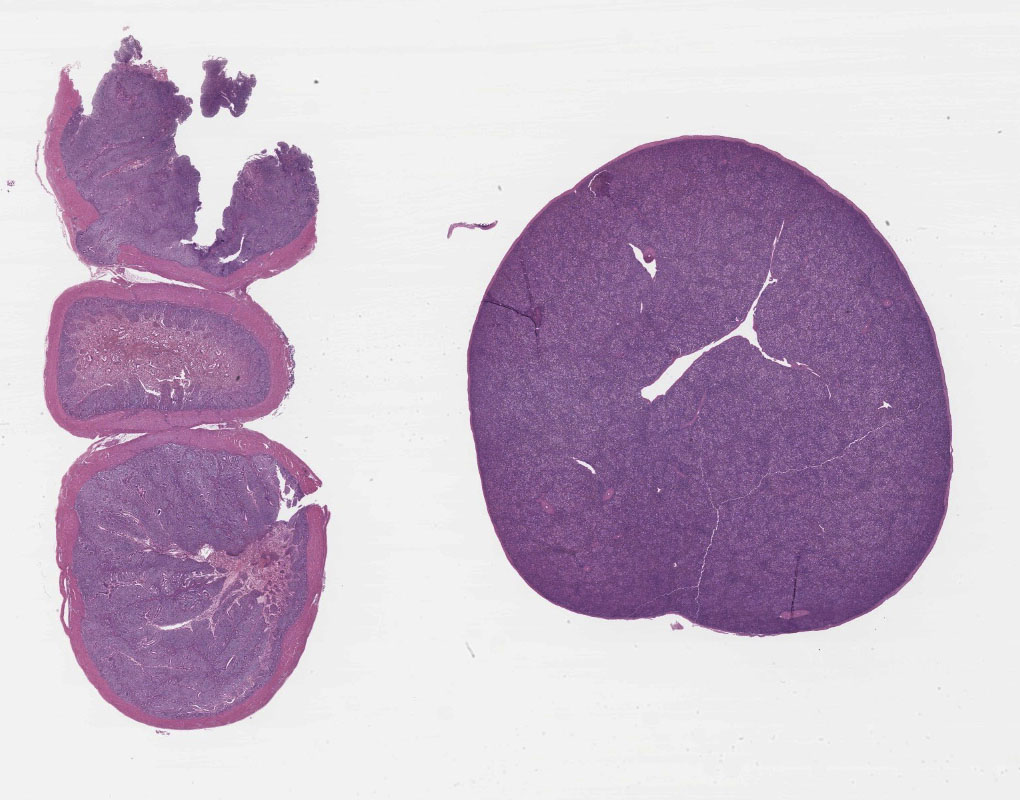
The intestinal tracts were distended, dark purple and filled with hemorrhagic contents and the intestinal mucosa was covered by a diphtheritic membrane. The spleens were enlarged with pale white mottling.
Laboratory Results:
Anaerobic Culture, Intestine: Clostridium perfringens, heavy growth
Aerobic Culture, Intestine: Salmonella sp. serotype infantis
Fecal Float: Eimeria, few
Microscopic Description:
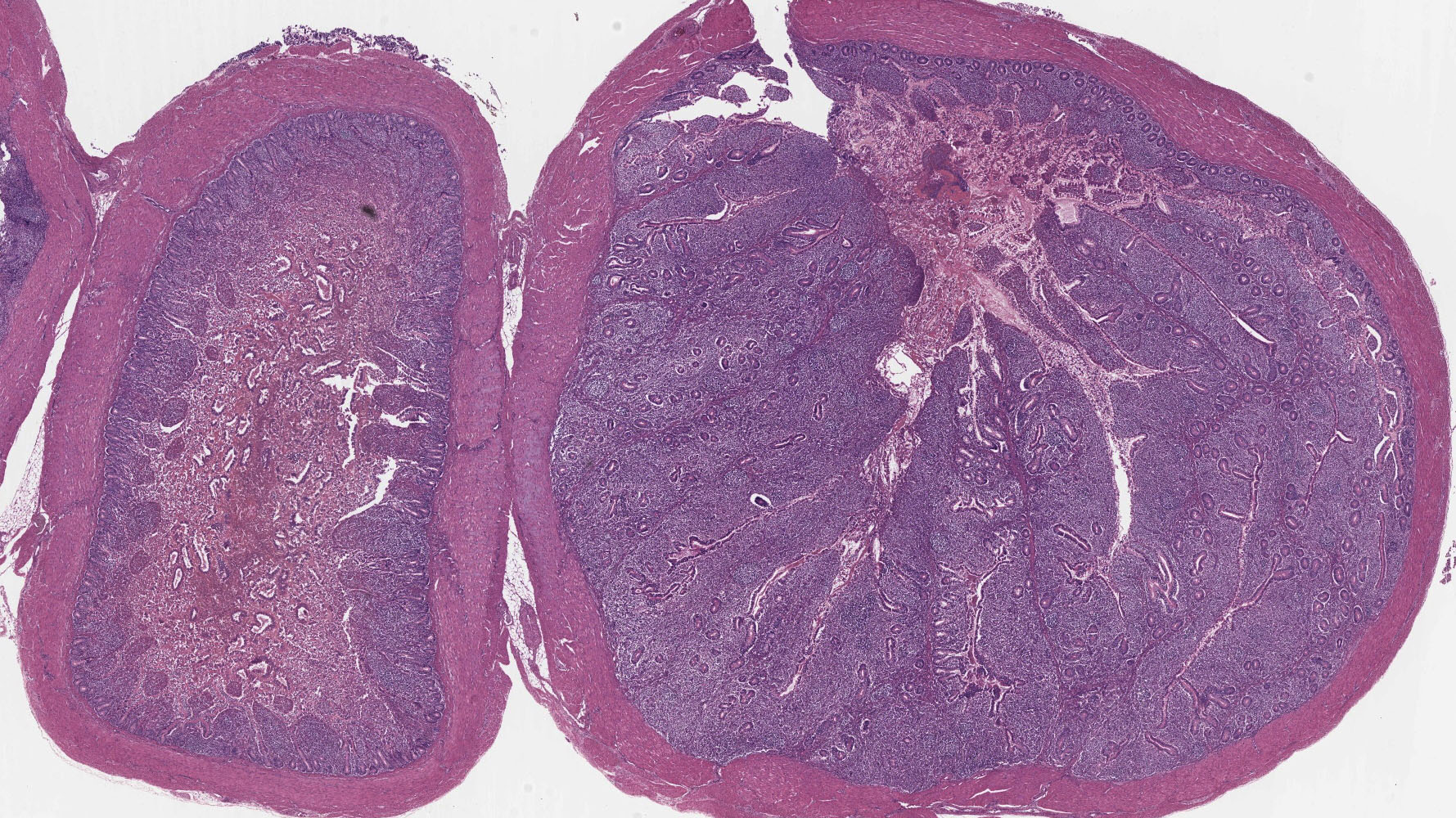
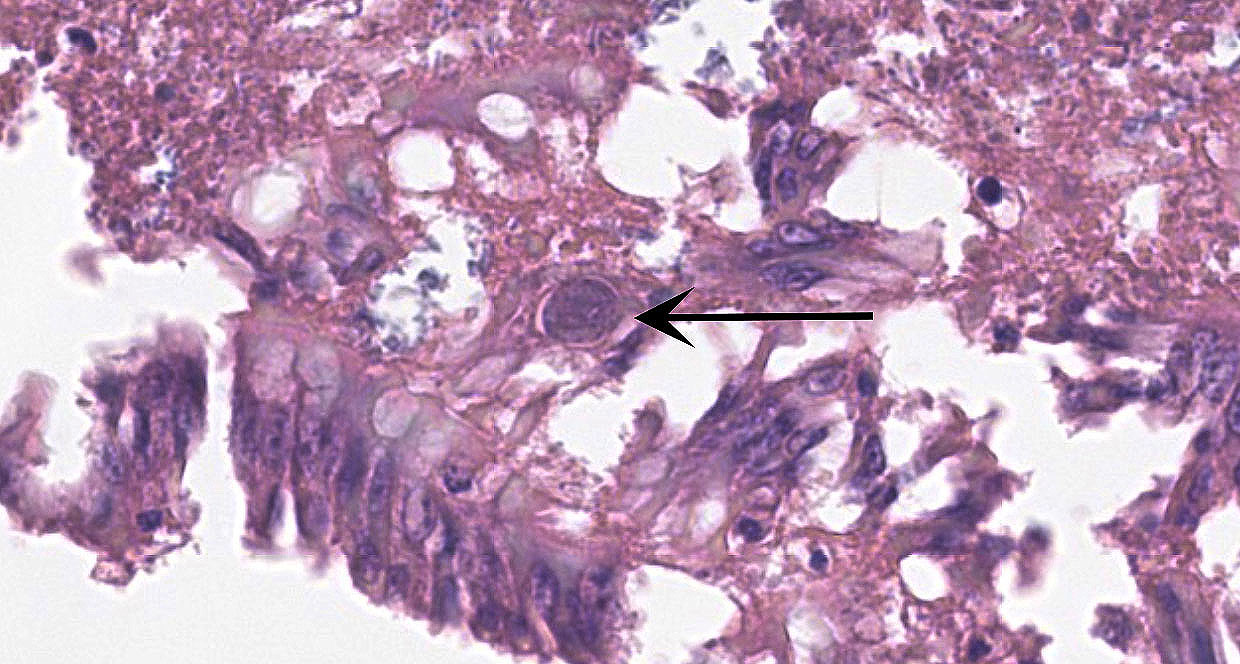
Small Intestine: In one section, villous architecture is obscured by hemorrhage diffusely expanding the lamina propria. Other tissue section depict simultaneous coagulative necrosis of villous tips with retention of villous outlines or partial to transmural mucosal ulcerative necrosis occasionally reaching the submucosa, mixed inflammatory reaction primarily lymphohistiocytic and heterophilic, intranuclear inclusions in the infiltrating lymphohistiocytic cells and Gram-positive rods colonizing the villus tips and areas of necrosis. There is widespread villous epithelial necrosis and sloughing into the lumina with villous atrophy, laminal proprial collapse, hemorrhage and fibrinous exudate admixed with myriad protozoal oocysts (Eimeria sp.) Oocysts have a thick wall and centrally located nucleus.
Ceca: Villous tips are lined by swollen or exfoliating pyknotic nuclei with attendant hemorrhage, fibrinous exudation, widespread necrotic destruction, and effacement of cecal tonsillar architecture by histiocytic infiltrate. Lymphohistiocytic cells in the laminal propria contain intranuclear inclusion bodies marginating nuclear chromatin. There is concomitant intense intra-epithelial parasitization by the various developmental stages of Eimeria sp. eliciting widespread destruction of villous and cryptal architecture with surface epithelial necrosis, erosion, ulceration and fibrin exudation, squamous metaplasia of regenerative surface epithelium, mucosal collapse, and infiltration of the lamina propria by myriad heterophils. There is bacterial invasion and colonization of surface epithelium.
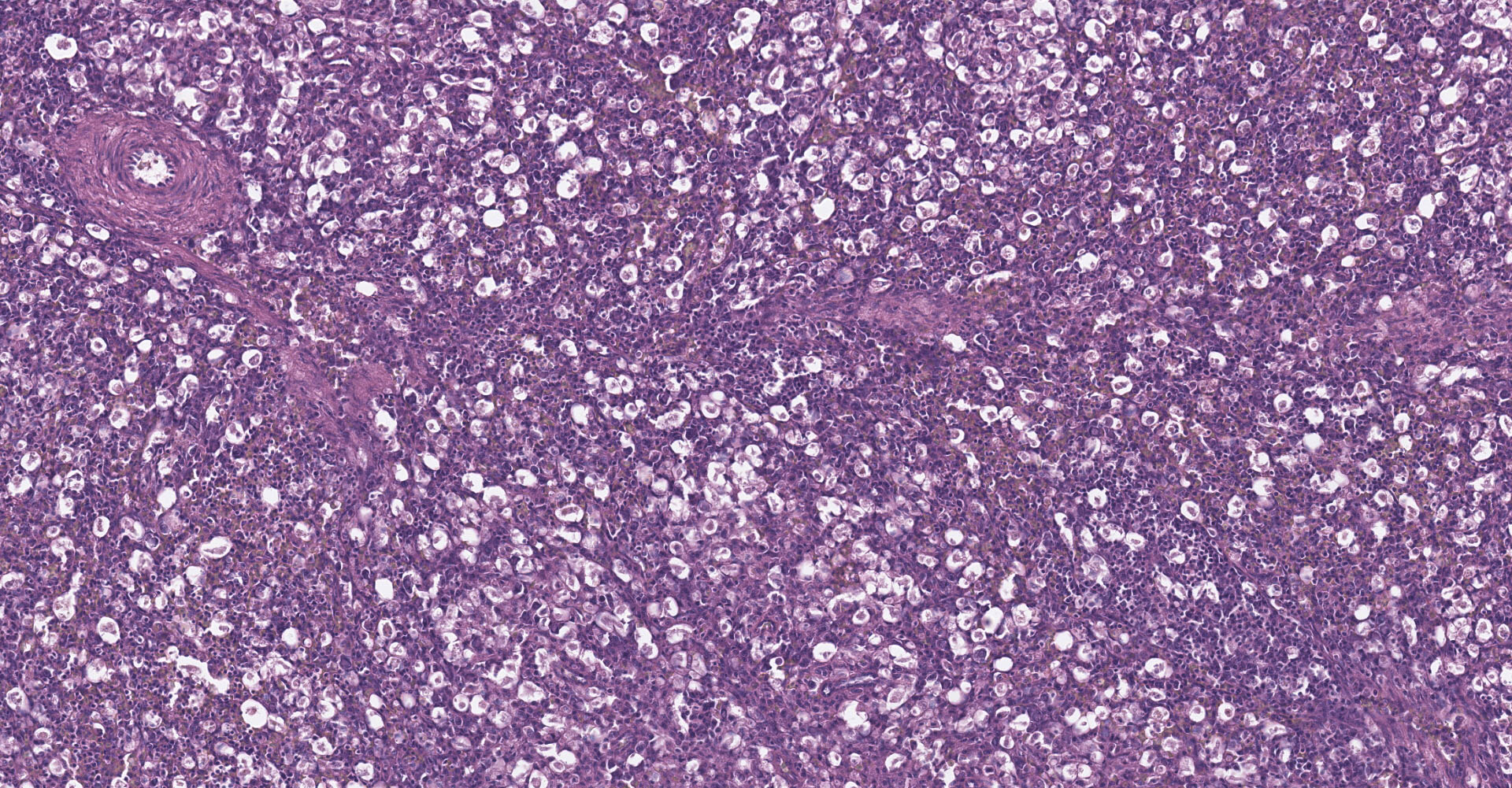
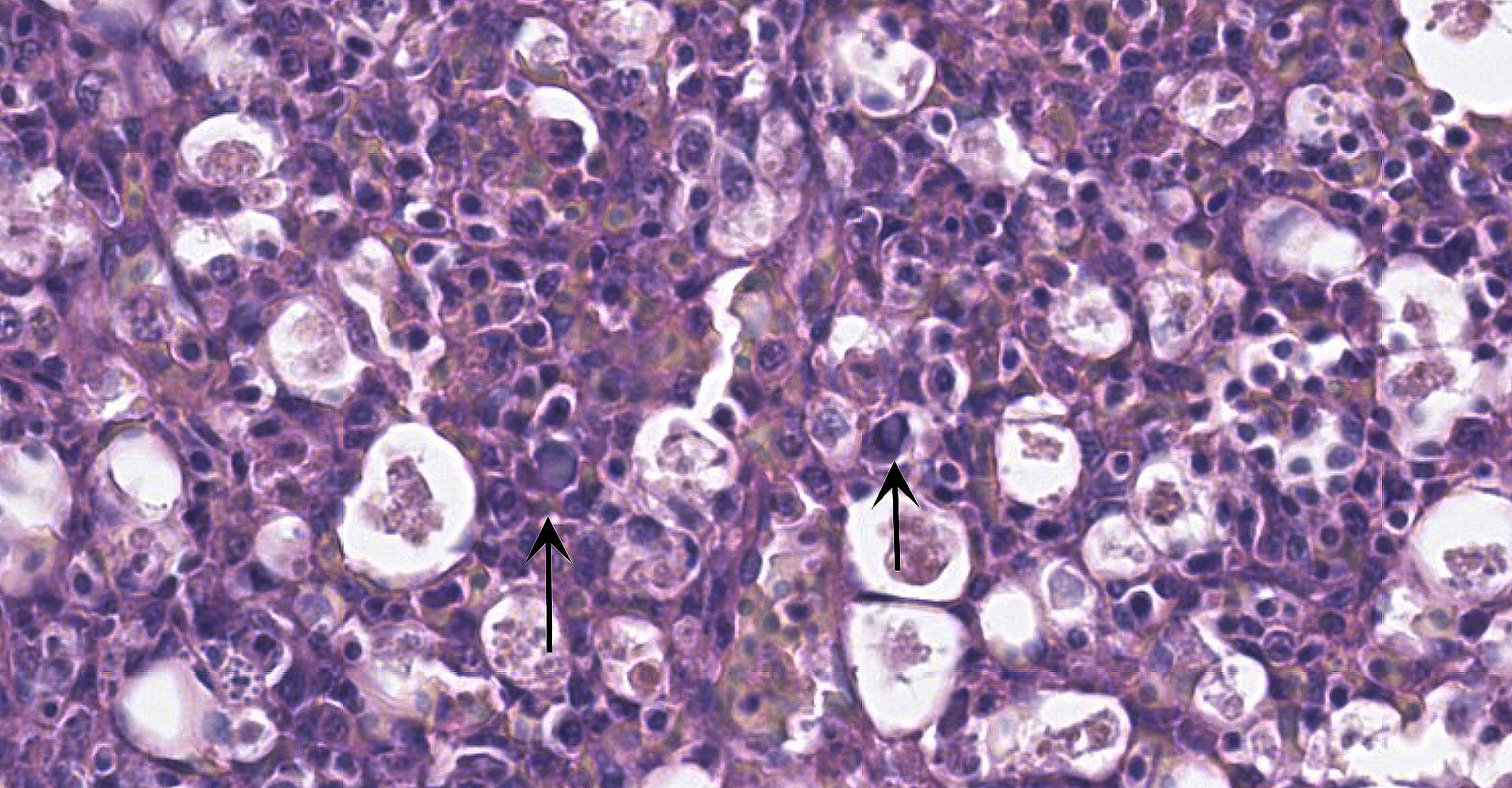
Spleen: There is widespread tissue pallor associated with necrosis and involution of white pulp and massive histiocytic infiltrate emanating from the sheathed arteries. Reticuloendothelial cells and infiltrating histiocytes contain large amphophilic to basophilic intranuclear inclusions with attendant chromatin margination characteristic for adenovirus and there are myriad tingible body macrophages imparting a "starry sky" appearance.
Contributor's Morphologic Diagnoses:
1. Spleen: Severe diffuse histiocytic splenitis with lymphoid depletion and intrahistiocytic intranuclear inclusions.
2. Small Intestine/Cecum: Severe hemorrhagic and necrotizing enterotyphlitis with intra-lymphohistiocytic intranuclear inclusions, intraepithelial protozoa, and intra-lesional bacteria.
Contributor's Comment:
Adenoviral infection with attendant transient immunosuppression facilitating opportunistic bacterial and protozoal colonization underlies the constellation of macroscopic and histologic lesions and disease trajectory in these birds. Adenoviral intranuclear inclusions visualized in lymphomononuclear cells in the intestinal and splenic tissues are characteristic for hemorrhagic enteritis (HE) of turkeys.
Hemorrhagic enteritis was first observed in 1936 in Minnesota turkeys ranging from 7-12 weeks of age and was described by Pomeroy and Fenstermacher.1 Although the birds were well-nourished and in good flesh, clinically affected birds were depressed with ruffled feathers and had watery discharge emanating from the vent.1 The entirety of the intestinal tract was visibly distended with mucosal congestion and serosanginous intestinal contents, however microscopic lesions were most striking in the duodenum.1 The principal early lesion involved erythrocytes and round cells infiltrating and thickening the lamina propria with the lesion progressing to exfoliation of villous epithelium and finally erosion and collapse of villous architecture.1 Later, the causative agent underlying HE in turkeys and marble spleen disease (MSD) of pheasants and avian adenovirus splenomegaly (AASV) of broiler breeders was attributed to adenoviral infection.2 HE of turkeys is associated with depression, splenomegaly, gastrointestinal hemorrhage, immunosuppression and death while MSD is primarily associated with respiratory disease in captive-raised pheasants.2
Utilization of adenoviruses as vectors for delivery of genes in vaccines and gene therapy prompted sequencing of the complete viral genome of the serologically indistinguishable 3 viruses. All 3 viruses were previously assigned to the genus Aviadenovirus, and designated as Group 2 aviadenoviruses.3 Genomic differences amongst the 3 viruses were minor although genomic sequence homologies indicated they were sufficiently different from other aviadenoviruses to warrant reassignment to a new genus Siadenovirus based upon their highly conserved sequential homology to the bacterial genes encoding for sialidase.4 Collectively these 3 viruses have been given the species name, Turkey adenovirus-3 (TAdV-3).4 HEV is a linear double-stranded DNA virus that is the smallest of any adenovirus thus characterized (26,263 bp) with low G+C content (34.93%).3 The HEV genome contains 8 ORFs bearing no similarity to ORFs described for adenoviruses.5 Several ORFs are arranged in two clusters-ORF 1,2,3,4 and ORF 7,8 with at least 13 genes encoding for structural proteins including the trimeric hexon, pentameric penton base, and trimeric fiber comprising the viral capsid.5 These three structural proteins are produced in large amounts and appear to be the most antigenic.6 Excepting minor genomic differences and host virulence factors, HEV and MSDV offer cross-protection directing vaccination strategies utilizing virulent MSDV strains in pheasants to vaccinate turkeys and strains of HEV virulent in turkeys to vaccinate pheasants.6 Phenotypic differences in clinical disease and lesion severity are influenced by sequential variations in the ORF1, E3 and fib genes.7
Maternal antibodies generally prevent infection in birds less than 6 weeks of age with fecal-oral/cloacal drinking the most likely exposure route for natural disease.8 To this end, prolonged environmental stability of viral particles in carcasses and wet fecal-contaminated litter mandate rigorous cleaning, disinfection and biosecurity practices.8
Upon oral exposure, absorbed viral particles target Ig-M bearing B-lymphocytes in the intestinal lamina propria and bursa of Fabricus, or enter the circulation homing towards the spleen, with viral replication targeting IgM+ B-lymphocytes and macrophages together with influx of CD4+ lymphocytes (acute infection) underlying white pulp hyperplasia and splenomegaly.9 HEV replication in IgM bearing B-lymphocytes and macrophages induces apoptosis and necrosis and reduced antigen presentation by lymphocytes and macrophages, offering two possible mechanistic explanations for HEV-induced immunosuppression leading to diminished humoral immunity against opportunistic infectious agents and vaccine failure.10,11 During peak infection, elaboration of Types I and II interferons and pro-inflammatory cytokines, namely IL-6 and TNF play competing protective and destructive roles.10,11 Elaboration of pro-inflammatory cytokines presumably modulates hemorrhagic disease because treatment with TNF antagonists prevents HEV-induced intestinal hemorrhage and diapedesis.10 The evolution of HEV-induced intestinal lesions owing to increased duodenal mast cells in response to cytokine elaboration by activated T cells is described.12
Given HEV tropism for IgM+ B-lymphocytes with attendant immunosuppressive effects, TAdV-3 infected birds are predisposed to secondary colibacillosis and may facilitate development of necrotic enteritis.13
In this case, the grower added Trimethoprim/Sulfa in the water for 7 days. The birds greatly improved, resuming normal water consumption and activity. The grower expressed concern about the viability of the initial HE vaccine. The vaccine should arrive frozen and stays frozen until thawed and mixed with a stabilizer over a 4 hour period. The grower indicated the 125,000 dose shipment and surrounding ice packs arrived already thawed. The vaccine was re-frozen and thawed again to be administered at both 17- and 27-day vaccines suggesting vaccine failure and inadequate protection.
Contributing Institution:
South Dakota State University Animal Disease Research and Diagnostic Laboratory
Box 2175, 1155 North Campus Drive
Brookings, SD 57007-1396
Telephone: (605)-688-5171
JPC Diagnosis:
1. Spleen: Splenitis, histiocytic, diffuse, severe with intranuclear viral inclusions, lymphoid depletion, and lymphocytolysis.
2. Ileum and cecum: Enterotyphlitis, histiocytic and lymphoplasmacytic, diffuse, severe with intranuclear viral inclusions.
3. Ileum and cecum: Intraepithelial apicomplexan schizonts, numerous with intraluminal oocysts.
JPC Comment:
The contributor provides an excellent review of Turkey adenovirus-3, the etiologic cause of hemorrhagic enteritis (HE), an economically significant disease most commonly seen in turkeys greater than 4 weeks of age.13
Although Pomeroy and Fenstermacher first described HE in 1937, it wasn't until 1967 that Gross and Moore discovered the etiologic agent was filterable through a 0.22 micron filter, supporting speculation of a viral entity. This suspicion was confirmed in 1974, when Carlson et al. identified adeno-like viral particles in tissues from affected birds.13
Introduction of HEV to susceptible flocks most often occurs as the result of exposure of naïve turkeys to contaminated feces or litter material. Lacking an envelope, TAdV-3 virons persist in the environment, retaining the ability to infect new hosts for up to 6 months at 4ºC and 4 years at -20ºC. In addition, virons are resistant to chemical inactivation via including quaternary ammonium compounds, ethyl ether, and zepharin chloride. However, exposure of the virus to high temperatures (>70ºC) for one hour or treatment with other agents such as 1% Lysol, 1% sodium lauryl sulfate, or sodium hypochlorite after removal of all organic material renders it non-infective.13
Although TAdV-3's environmental persistence has been established as a source of subsequent infection, recent findings have suggested recovered birds may also serve as asymptomatic carriers.13 Thus production facilities may risk re-contamination following post-outbreak sanitation measures if an affected flock is not depopulated.
As described by the contributor, apoptosis and necrosis of infected B-lymphocytes and macrophages results in impairment of both humoral and cell mediated immunity. Consequently, affected birds are not only more susceptible to secondary infection, but responses to vaccination are also suppressed, as has been demonstrated with low antibody titers following vaccination for Newcastle disease.13
Multiple entities cause similar gross splenic and gastrointestinal lesions in turkeys, including bacterial etiologies such as E. rhusiopathiae, P. multocida, E. coli, and Salmonella sp., as well as viral entities including Newcastle disease, avian influenza, reticuloendotheliosis, and lymphoprolif-erative disease. Histologically, the presence of adenoviral nuclear inclusions in lymphocytes and macrophages is diagnostic although they may not always be present. Additional diagnostics tests include agar gel immunodiffusion, antigen-capture ELISA, immunohistochemical staining, and immunofluorescence or immunoperoxidase methods. In addition, quantitative real-time PCR is a highly specific and sensitive method that can be particularly useful in outbreak scenarios as large numbers of samples can be processed in less time than when using conventional methods.13
Conference participants noted significant variation between slides in regard to the number of intraepithelial apicomplexans and intraluminal oocysts present within tissue sections.
References:
1. Pomeroy BS, Fenstermacher R. Hemorrhagic enteritis in turkeys. Poult Sci. 1937; 16(6):378-382.
2. Domermuth CH, Gross WB. Hemorrhagic enteritis and related infections. In: Hofstad MS, Barnes HJ, Calnek BW, Reid WM, Yoder JHW eds. Diseases of Poultry. 8th Ed. Ames, Iowa: Iowa State University Press; 1984:511-516.
3. Jucker MT, McQuiston JR, van den Hurk, JV, Boyle SM, Pierson FW. Characterization of the haemorrhagic enteritis virus genome and the sequence of the putative penton base and core protein genes. J. Gen. Virol. 1996; 77: 469?479.
4. Harrach BM, Benkö GW, Both M, et al. Virus taxonomy. Classification and nomenclature of viruses. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ eds. Ninth report of the international committee on taxonomy of viruses. San Diego, CA: Elsevier Academic Press; 2011:125-141.
5. Pitcovski J, Mualem M, Rei-Koren Z, et al. The complete DNA sequence and genome organization of the avian adenovirus, hemorrhagic enteritis virus. Virology. 1998; 249(2):307-315.
6. Pierson FW, Fitzgerald SD. Hemorrhagic enteritis and related infections. In: Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, Nair V eds. Disease of Poultry. 13th Ed. Ames, Iowa: Wiley Blackwell; 2013:309-331.
7. Beach NM, Duncan RB, Larsen CT, Meng XJ, Sriranganathan N, Pierson FW. Comparison of 12 turkey hemorrhagic enteritis virus isolates allows prediction of genetic factors affecting virulence. J Gen Virol. 2009; 90:1978-1985.
8. Pierson FW. Current thoughts on the pathogenesis of hemorrhagic enteritis (HE), HE-associated secondary bacterial infections, diagnosis, control, and prevention. In: Hafez HM ed. 11th "Hafez" International Symposium on Turkey Diseases. Berlin, Germany: Institute of Poultry Diseases, Free University Berlin; 2016:87-90.
9. Suresh M, Sharma JM. Hemorrhagic enteritis virus induced changes in the lymphocyte subpopulations in turkeys and the effect of experimental immunodeficiency on viral pathogenesis. Veterinary Immunol Immunopathol. 1995; 45(1-2):139-150.
10. Rautenschlein S, Sharma JM. Immunopathogenesis of haemorrhagic enteritis virus (HEV) in turkeys. Dev Comp Immunol. 2000; 24(2-3):237-246.
11. Rautenschlein S, Suresh M, Sharma J. Pathogenic avian adenovirus type II induces apoptosis in turkey spleen cells. Arch. Virol. 2000; 145:1671?1683.
12. Opengart K, Eyre P, Domermuth CH. Increased numbers of duodenal mucosal mast cells in turkeys inoculated with hemorrhagic enteritis virus. Am J Vet Res. 1992; 53(5):814-819.
13. Dhama K, Gowthaman V, Karthik K, et al. Haemorrhagic enteritis of turkeys ?
current knowledge, Veterinary Quarterly. 2017; 37(1):31-42.