Signalment:
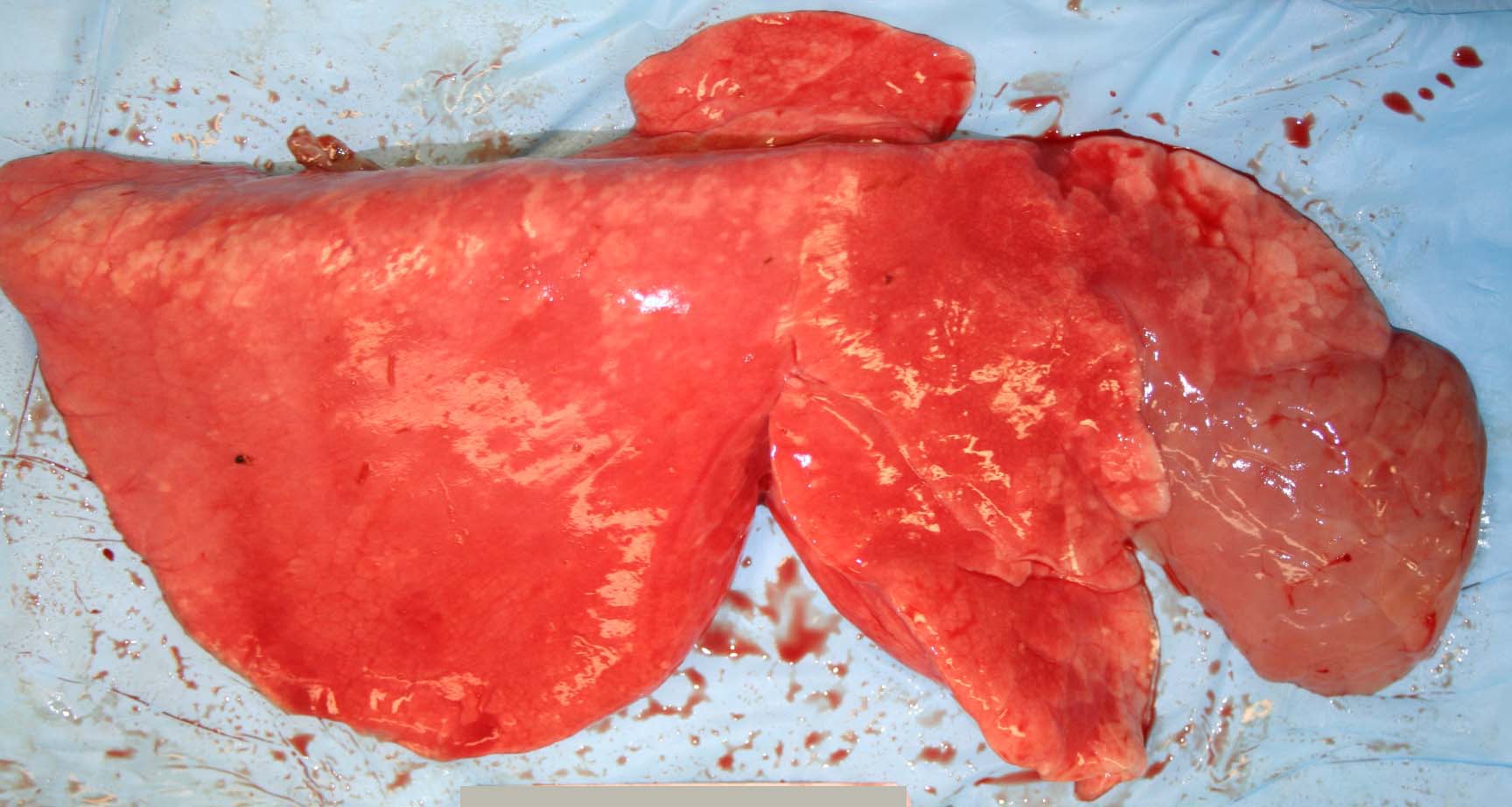
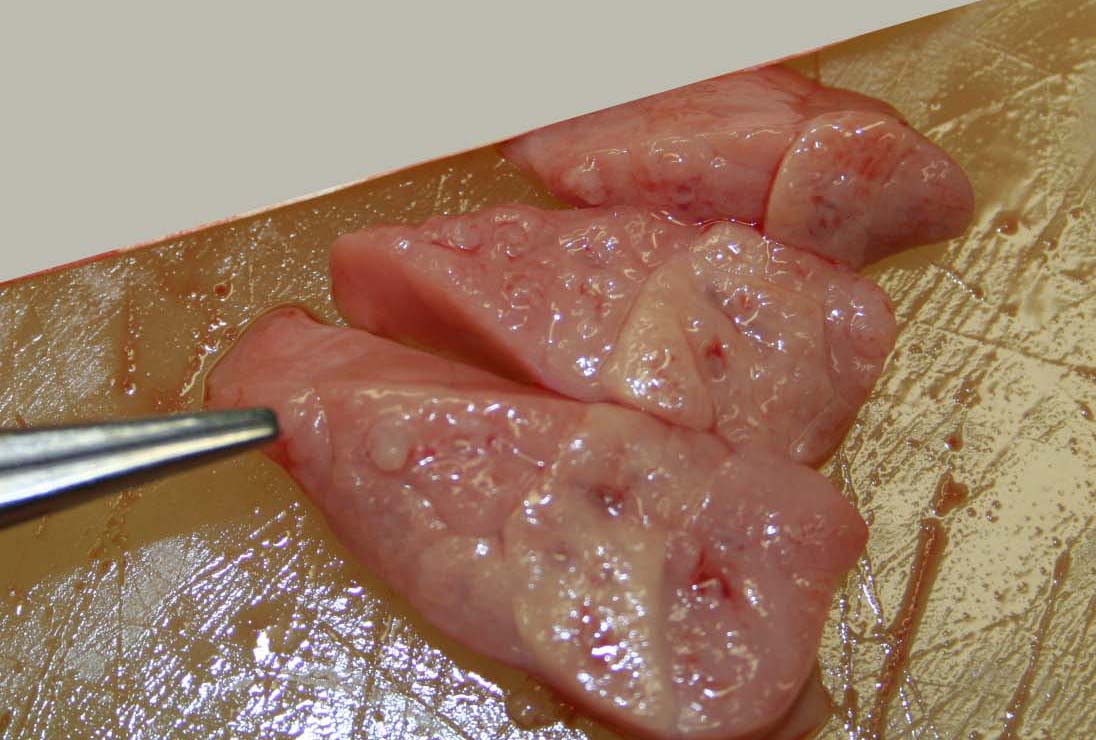
Gross Description:
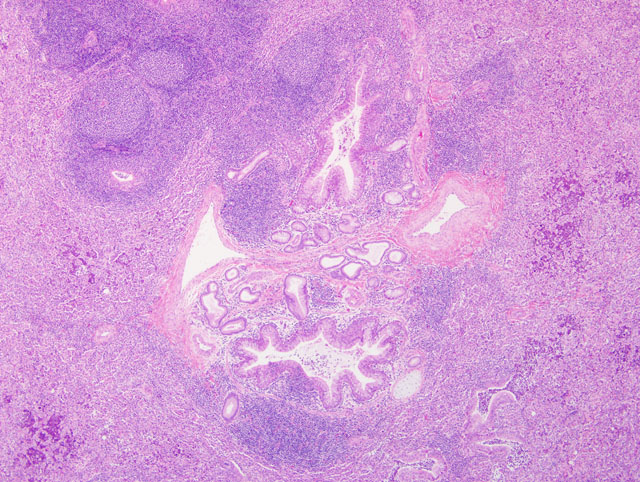
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Mycoplasma ovipneumoniae is considered the causative agent of atypical or chronic, non-progressive pneumonia, a mild or subclinical respiratory disease of lambs in the first year of life.(6) Mixing of animals, high stocking density, and poor air quality are considered to be important risk factors. Concurrent or secondary infection with Mannheimia haemolytica is frequently reported and contributes to suppurative inflammation.(6,11)
Experimentally, M. ovipneumoniae adheres to luminal ciliated epithelial cells and induces ciliostasis.(4,8) In one study, infection with M. ovipneumoniae was correlated with the development of autoantibodies to the cilia.(7) Additionally, M. ovipneumoniae has been shown to inhibit alveolar macrophage phagocytosis in vitro.(9) In this case, the presence of inspissated mucus casts, bronchiectasis, and mixed suppurative and histiocytic inflammation in the alveoli are strongly suggestive of impaired mucociliary clearance. Colonization of the ciliated epithelium with ciliostasis and deciliation are features of other Mycoplasma pneumonias.(2,5) Other virulence factors include alteration of host prostaglandin synthesis, membrane alterations, induction of apoptosis in lymphocytes and other cells, as well as the presence of superantigens in the mycoplasmal plasma membrane. This latter feature is thought to give rise to the typical lymphocytic cuffing that is a feature of many pulmonary mycoplasmoses.(2)
Mycoplasma ovipneumoniae has been implicated as a causative agent of enzootic and epizootic pneumonia in Rocky Mountain bighorn sheep (Ovis canadensis canadensis).(1) The gross and histologic lung lesions induced by M. ovipneumoniae are similar to that of M. pulmonis (and cilia-associated respiratory bacillus) in laboratory rats (and mice)(10) and M. hyopneumoniae in pigs,(2,5) but differ markedly from the typical appearance of Mycoplasma bovis pneumonia in cattle.(2,3)
JPC Diagnosis:
Conference Comment:
Conference participants reviewed and discussed the three primary respiratory tract defense mechanisms: 1) mucus and the mucociliary escalator system; 2) antibody and innate defense proteins; and 3) alveolar macrophages and recruited neutrophils. Hairs and air turbulence in the nasal passages and sinuses prevent particles larger than 10 μm in size from advancing further in the respiratory tract. Particles entrapped in mucus are cleared via the mucociliary escalator and/or coughing. The mucociliary escalator ends at the level of the terminal bronchioles, making this anatomic area in the respiratory tree the most susceptible to establishment of infection. The airway mucosa contains many non-specific antimicrobial factors, including lactoferrin, B-defensins, collectins, lysozyme, the complement membrane attack complex and lactoperoxidase, among others. Opsonizing agents in the respiratory tract include IgG, C3b from the complement system and surfactant proteins A and D. Primary defense and clearing of debris in the alveolus is accomplished by alveolar macrophages; T lymphocytes and natural killer cells assist in sampling alveolar material.(2) Conference participants discussed the importance of impaired mucociliary clearance and ciliostasis in the pathogenesis of myocoplasmal pneumonia, and noted additional causes of ciliostasis, including cilia-associated respiratory bacillus (CAR bacillus) of rodents, Bordetella bronchiseptica, alcohol, smoking, and primary ciliary dyskinesia.
References:
2. Caswell JL, Williams, KJ: Respiratory System. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, ed. Maxie MG, 5th ed., pp. 579-650. Elsevier Saunders, Philadelphia, PA, 2007
3. Gagea MI, Bateman KG, Shanahan RA, van Dreumel T, McEwen BJ, Carman S, Archambault M, Caswell JL: Naturally occurring Mycoplasma bovis-associated pneumonia and polyarthritis in feedlot beef calves. J Vet Diagn Invest 18:29-40, 2006
4. Jones GE, Keir WA, Gilmour JS: The pathogenicity of Mycoplasma ovipneumoniae and Mycoplasma arginini in ovine and caprine tracheal organ cultures. J Comp Pathol 95:477-487, 1985
5. Lopez A: Respiratory System. In: Pathologic Basis of Veterinary Disease, ed. McGavin M, Zachary, JF, 4th ed., pp. 463-558. Mosby Elsevier, St. Louis, MO, 2007
6. Martin WB: Respiratory infections of sheep. Comp Immunol Microbiol Infect Dis 19:171-179, 1996
7. Niang M, Rosenbusch RF, Andrews JJ, Lopez-Virella J, Kaeberle ML: Occurrence of autoantibodies to cilia in lambs with a 'coughing syndrome'. Vet Immunol Immunopathol 64:191-205, 1998
8. Niang M, Rosenbusch RF, DeBey MC, Niyo Y, Andrews JJ, Kaeberle ML: Field isolates of Mycoplasma ovipneumoniae exhibit distinct cytopathic effects in ovine tracheal organ cultures. Zentralbl Veterinarmed A 45:29-40, 1998
9. Niang M, Rosenbusch RF, Lopez-Virella J, Kaeberle ML: Expression of functions by normal sheep alveolar macrophages and their alteration by interaction with Mycoplasma ovipneumoniae. Vet Microbiol 58:31-43, 1997
10. Percy D, Barthold, SW: Pathology of Laboratory Rodents and Rabbits, 3rd ed. pp. 65-67, 143-146. Blackwell Publishing, Ames, IA, 2007
11. Sheehan M, Cassidy JP, Brady J, Ball H, Doherty ML, Quinn PJ, Nicholas RA, Markey BK: An aetiopathological study of chronic bronchopneumonia in lambs in Ireland. Vet J 173:630-637, 2007