WSC 22-23
Conference 21
Case IV:
Signalment:
An 8-month-old spayed female Boxer dog (Canis familaris)
History:
The dog was referred by a practitioner to a specialist veterinary center for management of acute spontaneous pneumothorax. On presentation to the specialist clinic the dog was tachypneic (64 bpm), hypoxemic (pulse oximetry 66% without oxygen supplementation, 92% with oxygen supplementation), and had increased respiratory effort with an abdominal component to the respiration. Lung sounds on the right side were dull on auscultation. Initial bloodwork revealed a mixed acidosis (pH 7.299; normal 7.35 - 7.45), with mild hemoconcentration (57%) and normal total solids (6.6 g/dl). Blood glucose was normal (4.7 g/dl), with no evidence of lactic acidosis (1.9 mmol/L; normal < 2.5 mmol/L).
Computed tomography (CT) showed multiple variably sized bullae in the right middle lung lobe, and partial or complete atelectasis of all remaining lung lobes. The dog was anesthetized for surgery but died from cardiopulmonary arrest during pre-surgical thoracocentesis.
Gross Pathology:
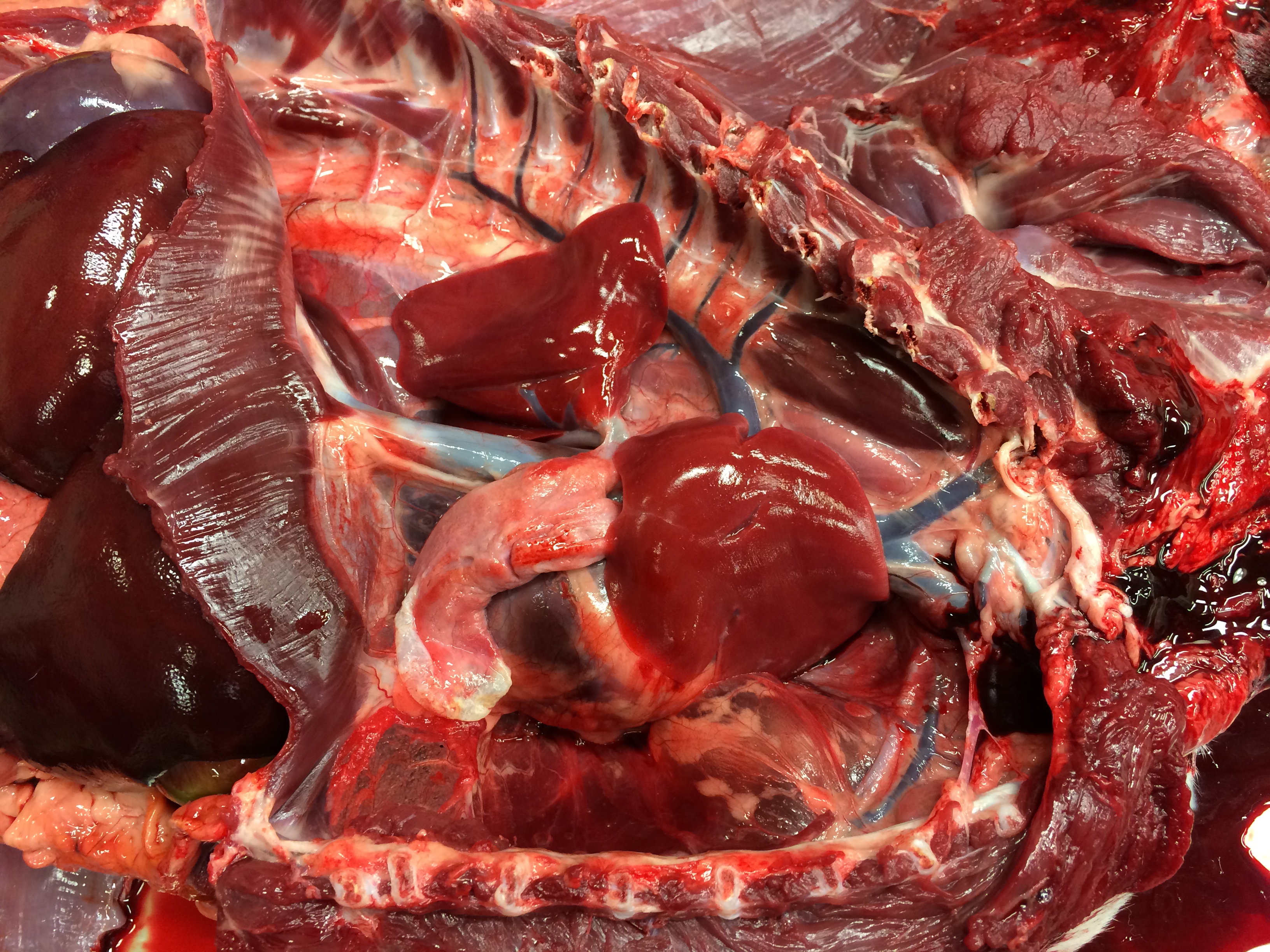
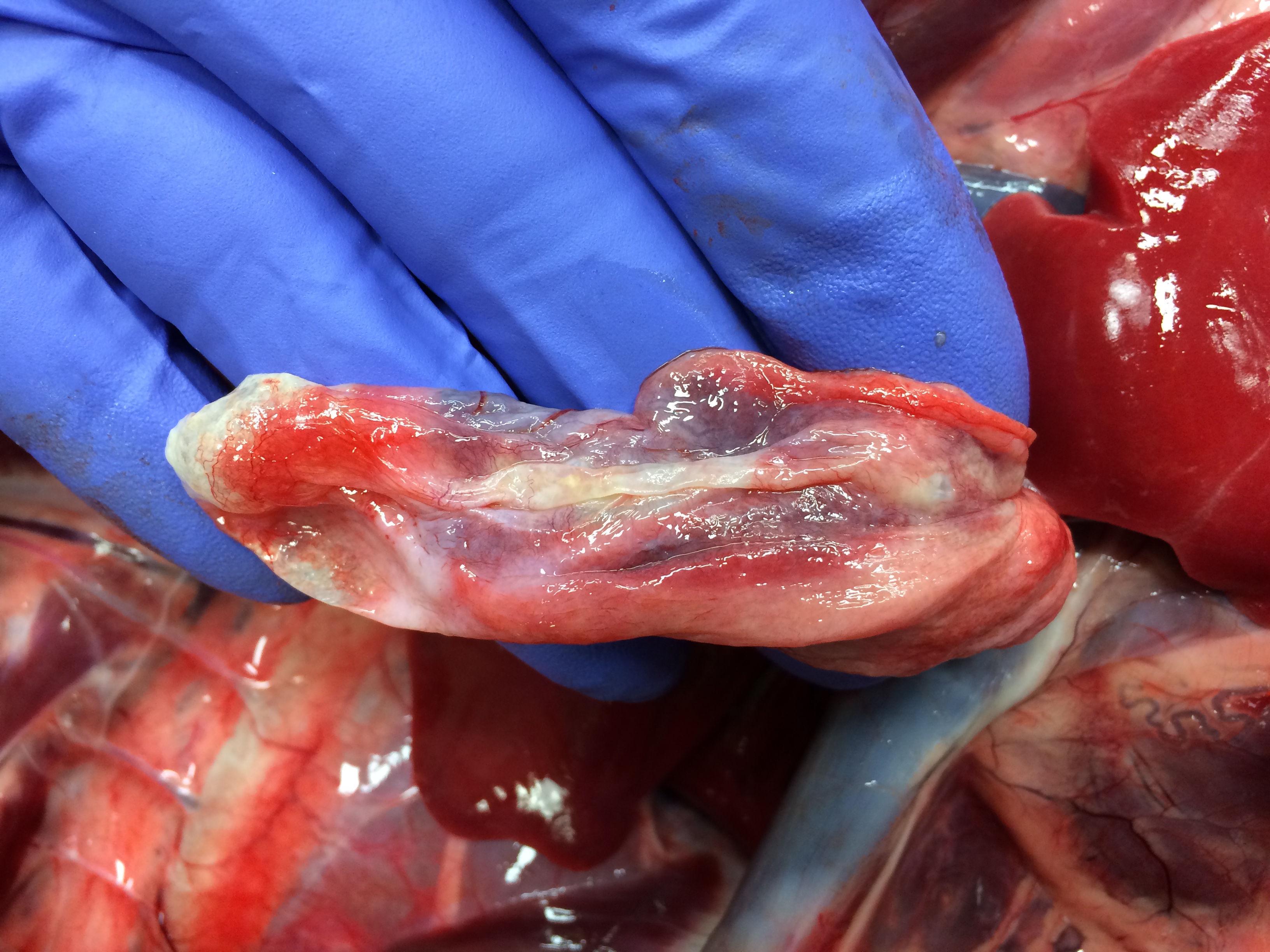
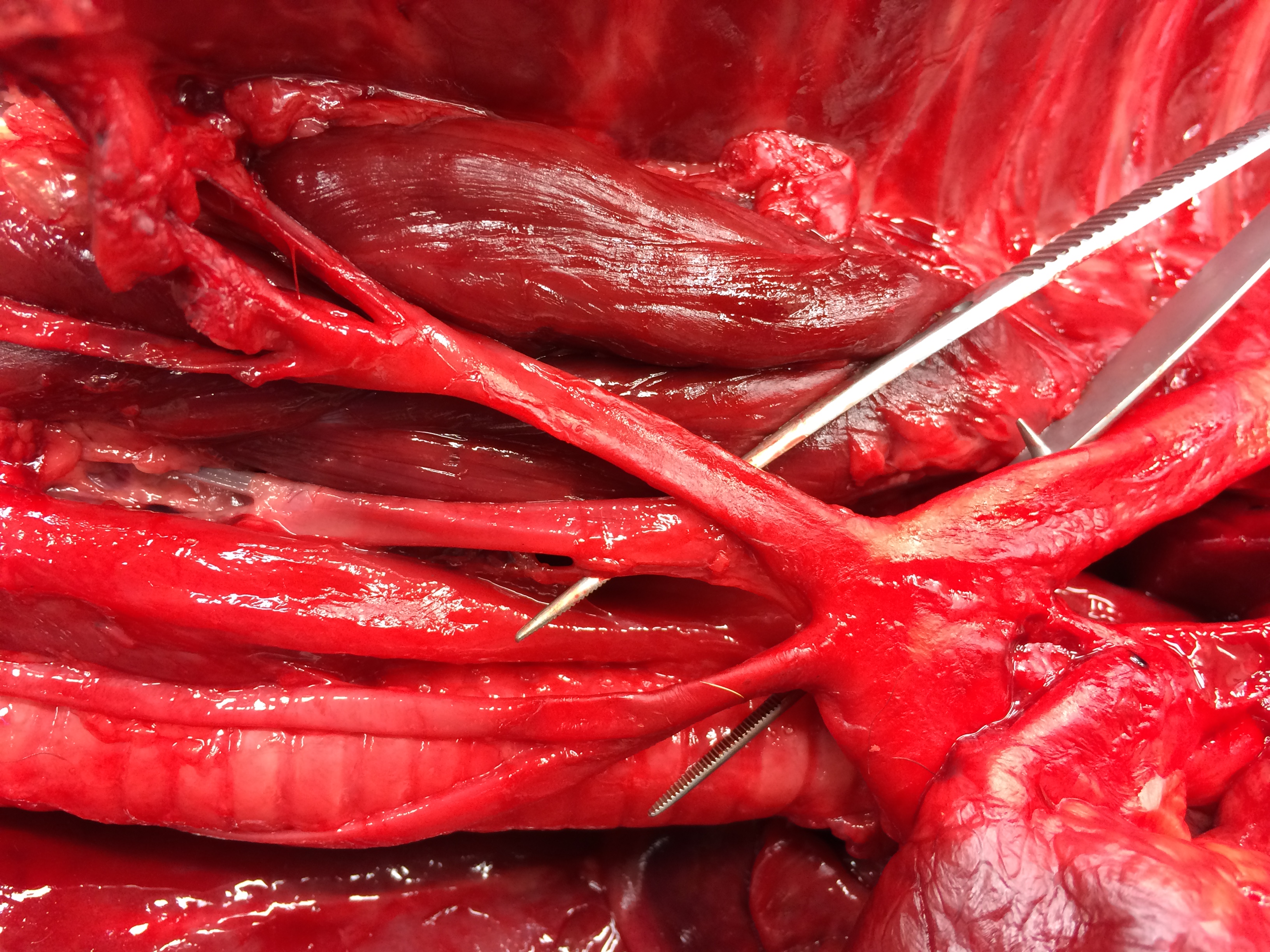
Necropsy examination revealed severe bilateral pneumothorax, with marked atelectasis of all lung lobes. The right middle lung lobe was small, pale and flaccid, with numerous collapsed, coalescing pleural bullae along its ventral and ventrocaudal margins; rupture of these bullae was presumed to be the source of the pneumothorax. The cartilage of the lobar bronchus supplying the right middle lung lobe was thin, flimsy and easily compressed when compared with bronchi supplying other lobes. In transverse section its lumen was slit-shaped rather than circular. In addition, an aberrant right subclavian artery arose in common with the left subclavian artery, forming a bisubclavian trunk. The right subclavian artery passed dorsal to the esophagus and compressed it.
Laboratory Results:
No laboratory results reported.
Microscopic Description:
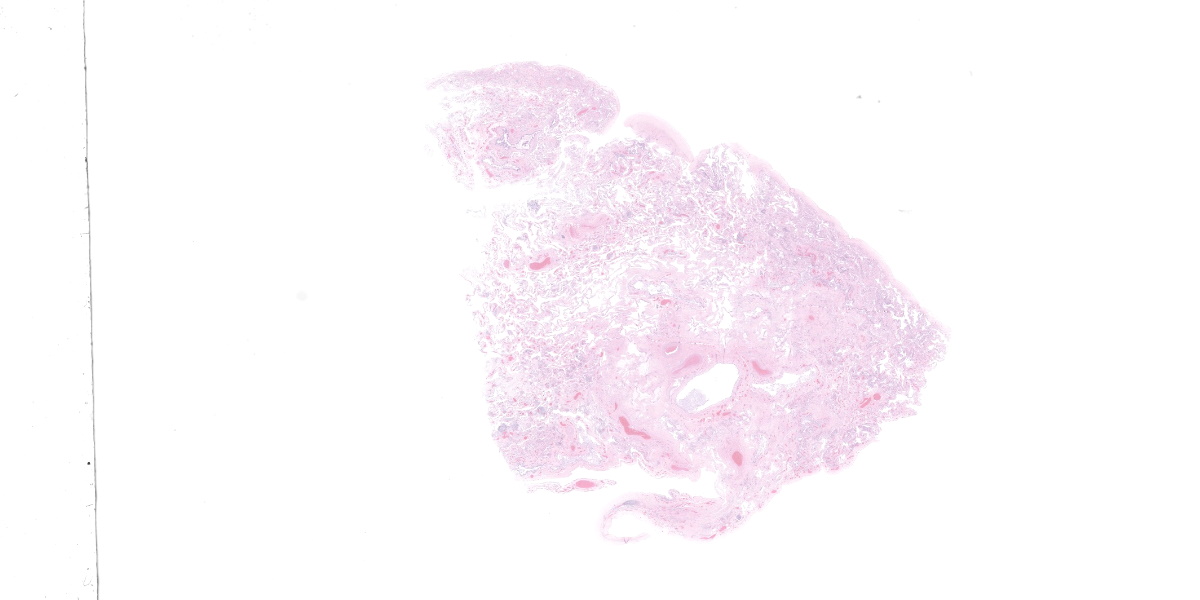
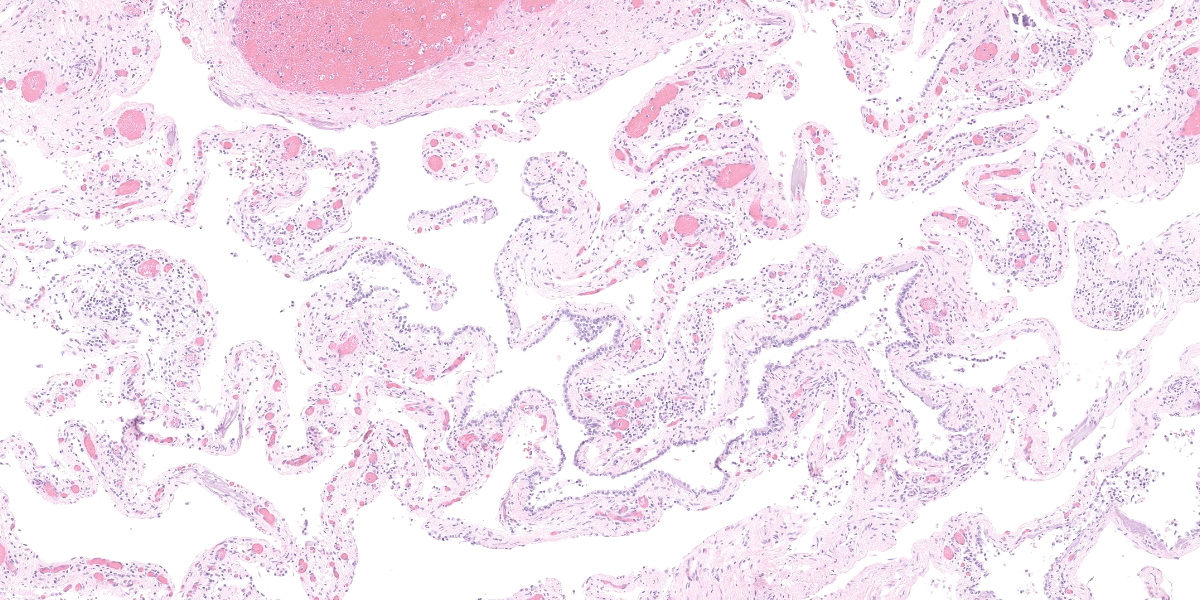
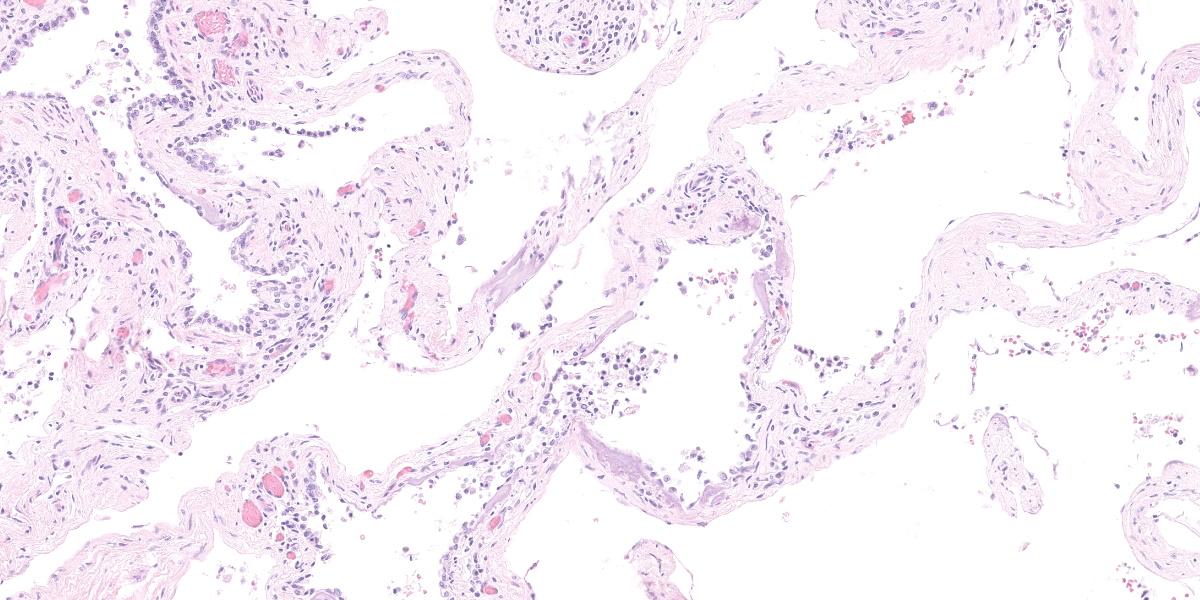
This section from the right middle lung lobe is severely malformed and difficult to recognize as lung. It consists predominantly of a thick fibrovascular trabecular network outlining collapsed; empty spaces lined by a simple cuboidal to simple squamous epithelium. The lining epithelium is multifocally lost (autolytic artifact), and cystic spaces frequently contain rafts of sloughed epithelial cells. The fibrovascular trabeculae are variably thick, ranging in width from 20 to 300 microns, and contain many thin-walled blood vessels lined by mature endothelial cells, numerous mineralized areas, and scattered lymphoplasmacytic foci.
Multifocally, recognizable bronchiole-like structures are present. These are lined by simple cuboidal epithelium and have irregular bundles of smooth muscle in their walls. They are frequently adjacent to profiles of large arteries and veins (pulmonary vessels).
The section also contains a malformed bronchus surrounded by a nearly continuous layer of smooth muscle. Cartilage plates in this bronchus are irregular and present only around one half of the airway’s circumference. The cartilage is immature, with small chondrocytes, less chondroid matrix and smaller lacunae (when compared with other lung sections from this dog and an age-matched control dog). Bronchial glands are fewer in number (when compared with other lung sections from this dog and the control dog).
Contributor’s Morphologic Diagnoses:
Lung: Congenital pulmonary airway malformation (CPAM)-like lesion
Contributor’s Comment:
The differential diagnosis for a single malformed lung lobe in a dog includes congenital lobar emphysema (CLE), a congenital pulmonary sequestration, and congenital pulmonary airway malformation (CPAM). Based on gross and histologic findings, the first two potential diagnoses can be ruled out in this dog. CLE requires overinflated alveoli or hyperplastic alveoli, whereas the affected lobe in this dog lacks alveoli. A congenital pulmonary sequestration lacks, by definition, any connection with the tracheobronchial tree. In this dog the affected lobe had a lobar bronchus, although it was flattened. Therefore, a presumptive diagnosis of CPAM-like lesion was made based on the similarity of this dog’s lesions to described human lesions of CPAM and exclusion of other potential diagnoses.
The term CPAM refers to a spectrum of human airway malformations characterized by abnormal development of various portions of the tracheobronchial tree.16 Formerly called congenital cystic adenomatoid malformations, CPAMs are well-recognized and relatively common in people. All types of CPAM are characterized by multiple irregular pulmonary cystic structures lined by varying types of epithelium. CPAMs in people are generally subdivided into five types based on the anatomic site of the malformation.16 These are the: proximal tracheobronchial tree (type 0); bronchial/proximal bronchiolar region (type 1); bronchiolar region (type 2); terminal bronchiolar/alveolar duct region (type 3); and alveoli (type 4). There may be overlap between different types, and other classification systems have been proposed.11,13
The malformations in the dog in this case report shared overlapping features of human CPAM types 2 and 4. Type 2 CPAM is characterized by the presence of numerous 2-15 mm diameter parenchymal cysts. These consist of dilated bronchiole-like structures lined by cuboidal to columnar epithelium but with mucus-producing cells generally absent. Other congenital abnormalities, including cardiovascular and renal malformations may be present in humans. (Interestingly, this dog had a malformation of the right subclavian artery, although the clinical significance of this was uncertain.) Type 4 CPAM is characterized by larger cysts, typically found toward the periphery of the lobe. These are lined by a mixture of flattened or rounded alveolar lining cells, and occasionally a low cuboidal epithelium. Both type 2 and type 4 human CPAMs typically affect a single lung lobe, as in this dog, and have a good prognosis after surgical resection.16,17
Like CPAM, congenital lobar emphysema (CLE) is also a well-recognized congenital lung malformation in humans.7,8,17 It is characterized by overinflation of a single lung lobe due to partial obstruction of the bronchus supplying that lobe.10 This obstruction can result from numerous causes, including malformation of the bronchus, external compression of the bronchus by masses or vascular anomalies, or internal obstruction of the bronchial lumen by mucus plugs or granulation tissue.8 Regardless of the cause, the result is significant lobar overinflation as the partially obstructed bronchus acts as a one way valve and traps air. In the dog described in this case report the lobar bronchus was malformed and partially collapsed, consistent with one cause of CLE. However, in human CLE, alveoli are distended but are otherwise histologically normal, and fibrosis, inflammation or necrosis are rarely seen.8 This is significantly different from the histologic lung changes in the dog described in this case report.
CLE has been previously reported several times in dogs,1,5,6,11,12,14,15,19,20 and the dog described in this report has several features in common with many reported canine cases of CLE: the right middle lung lobe was affected; a bronchial structural abnormality was present; bullae and pneumothorax were a feature; and the dog was less than six months old. However, this dog’s most significant histologic lesions (absence of recognizable alveolar architecture, with marked interstitial fibrosis) were incompatible with the simple alveolar hyperinflation expected in CLE. Seven human pediatric pathologists were consulted on this case and all agreed that this dog did have a congenital lung malformation and that it was not CLE.
This case highlights that there are multiple types of congenital lung malformations in dogs. It is possible that some reported canine cases of CLE may, in fact, represent CPAM-like lesions. Among published canine CLE reports, two describe histologic features that are suggestive of CPAM type 24 or type 4.11 It may be that these were cases of CPAM-like lesions that were interpreted to be CLE, or that forms of pulmonary disease that share features of both diseases exist in dogs. There are numerous reports of human congenital lung lesions fulfilling criteria for both CPAM and other congenital lung lesions,7 and this may also prove to be the case in dogs.
It is important to recognize that not all congenital lung malformations in dogs are CLE, and that this diagnosis should not be made based solely on imaging or the gross appearance of an affected lung lobe; CPAM and CLE must be distinguished histologically. In humans there are practical clinical reasons for making an accurate diagnosis of congenital lung lesions. Most importantly, CPAM may progress to malignant neoplasia while CLE does not.5,7 Lobectomy is generally indicated for CPAM lesions, while CLE may resolve spontaneously and can often be managed conservatively unless it causes mediastinal displacement or severe dyspnea.7 Because CPAM-like disease in dogs has not been reported previously it is not possible to compare its treatment or prognosis with those of CLE.
Contributing Institution:
Department of Veterinary Clinical and Diagnostic Sciences
Faculty of Veterinary Medicine
University of Calgary
3280 Hospital Dr. NW
Calgary, Alberta T2N 4Z6
Canada
http://vet.ucalgary.ca/
JPC Diagnosis:
Lung: Congenital pulmonary airway malformation with mineralization.
JPC Comment:
The contributor provides an excellent review of congenital pulmonary airway malformations, congenital lobar emphysema, and the differences between the two diagnoses.
This week’s moderator described the six stages of development of the lung: embryonic, pseudoglandular, canalicular, saccular, alveolar, and vascular maturation. Additionally, the moderator described that TTF-1 and HNF-3 are important transcription factors for early lung development.
Congenital pulmonary abnormalities in veterinary species are rare, and one of the more common conditions is accessory lung development in ruminants.2,9 Accessory lungs are found in extrapulmonary locations, such as in the abdomen and subcutis.2,9 These lobulated masses consist of haphazardly arranged and variably differentiated pulmonary elements, including bronchi lined by ciliated respiratory epithelium, alveoli, cartilage, and blood vessels.2,9 While composed of similar elements, pulmonary hamartomas are different in that they are located within the lung. Pulmonary hamartomas are the most commonly diagnosed benign lung tumor in humans, typically discovered as incidental findings on radiographs, and are also occasionally seen in ruminants.2,3 A recent article described a pulmonary hamartoma in a captive-bred, full-term elk calf found dead. The mass replaced the left caudal lung lobe, encompassed approximately half of the thorax, and compressed the remaining lungs.2
The moderator and conference participants also remarked on the presence of mineralization in this case; an explanation for mineralization was not readily apparent on H&E examination.
References:
- Billet JPHG, Sharpe A. Surgical treatment of congenital lobar emphysema in a puppy. J Small Anim Pract. 2002; 43: 84-87.
- Boggiatto PM, Steven SC, Palmer MV. Pulmonary hamartoma in an elk calf. J Vet Diagn Invest. 2023; 3; 5(2): 193-195.
- Caswell JL, Williams KJ. Respiratory System. In: Grant MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th St. Louis, MO: Elsevier. 484-485.
- Gopalakrishnan G, Stevenson GW. Congenital lobar emphysema and tension pneumothorax in a dog. J Vet Diagn Invest. 2007; 19: 322-325.
- Hancock BJ, Dilorenzo M, Youssef S, Yazbeck S, Marcotte JE, Collin PP. Childhood Primary Pulmonary Neoplasms. J Pediatr Surg. 1993; 28: 1133-1136.
- Herrtage ME, Clarke DD. Congenital lobar emphysema in 2 dogs. J Small Anim Pract. 1985; 26: 453-464.
- Laberge J-M, Puligandla P, Flageole H. Asymptomatic congenital lung malformations. Semin Pediatr Surg. 2005; 14: 16-33.
- Langston C. New concepts in the pathology of congenital lung malformations. Semin Pediatr Surg. 2003; 12: 17-37.
- Lopez A, Martinson SA. Respiratory System, Thoracic Cavities, Mediastinum, and Pleura. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th St. Louis, MO: Elsevier. 2022; 570.
- Mani H, Suarez E, Stocker JT. The morphologic spectrum of infantile lobar emphysema: a study of 33 cases. Paediatr Respir Rev. 2004; 5: S313-S320.
- Matsumoto H, Kakehata T, Hyodo T, Hanada K, Tsuji Y, Hoshino S, Isomura H. Surgical correction of congenital lobar emphysema in a dog. J Vet Med Sci. 66: 217-219.
- Moon H-S, Lee M-H, Han J-H, Kim S-K, Lee S-G, Lee L, Hyun C. Asymptomatic congenital lobar emphysema in a Pekinese dog. Journal of Animal and Veterinary Advances. 2007; 6: 556-558.
- Puligandla PS, Laberge JM: Congenital Lung Lesions. Clin Perinatol. 2012; 39: 331-347.
- Ruth J, Rademacher N, Ogden D, Rodriguez D, Gaschen L. Imaging diagnosis—congenital lobar emphysema in a dog. Vet Radiol Ultrasound. 2011l 52: 79-81.
- Stephens JA, Parnell NK, Clarke K, Blevins WE, DeNicola D. Subcutaneous emphysema, pneumomediastinum, and pulmonary emphysema in a young schipperke. J Am Anim Hosp Assoc. 2002; 38: 121-124.
- Stocker J. Congenital pulmonary airway malformation: a new name for and an expanded classification of congenital cystic adenomatoid malformation of the lung. Histopathology. 2002; 41: 424-431.
- Stocker JT. Congenital and Developmental Diseases. In: Tomashefski Jr JF, ed. Dail and Hammar’s Pulmonary Pathology. 3rd New York: Springer Science. 2008.
- Tennant BJ, Haywood S. Congenital bullous emphysema in a dog - a case report. J Small Anim Pract. 1987; 28: 109-116.
- Voorhout G, Goedegebuure SA, Nap RC. Congenital lobar emphysema caused by aplasia of bronchial cartilage in a Pekingese puppy. Vet Pathol. 1986; 23: 83-84.
- Yun S, Lee H, Lim J, Lee K, Jang K, Shiwa N, Boonsriro H, Kimitsuki K, Park C, Kwon Y. Congenital lobar emphysema concurrent with pneumothorax and pneumomediastinum in a dog. J Vet Med Sci. 2016; 78: 909-912.