CASE III: N19-705A (JPC 4152931)
Signalment:
Adult, 18 kg, intact male North American opossum (Didelphis virginiana), marsupial
History:
The opossum was submitted from the wildlife center. The patient presented for emaciation (body condition score 2/9) and multiple abrasions in the skin of the right aspect of the chin, tail base and bilaterally on the carpal paw pad, as well as a deep puncture wound in the right chin that extended into the oral cavity. The opossum declined clinically and displayed marked respiratory distress;
euthanasia was performed due to poor prognosis.
Gross Pathology:
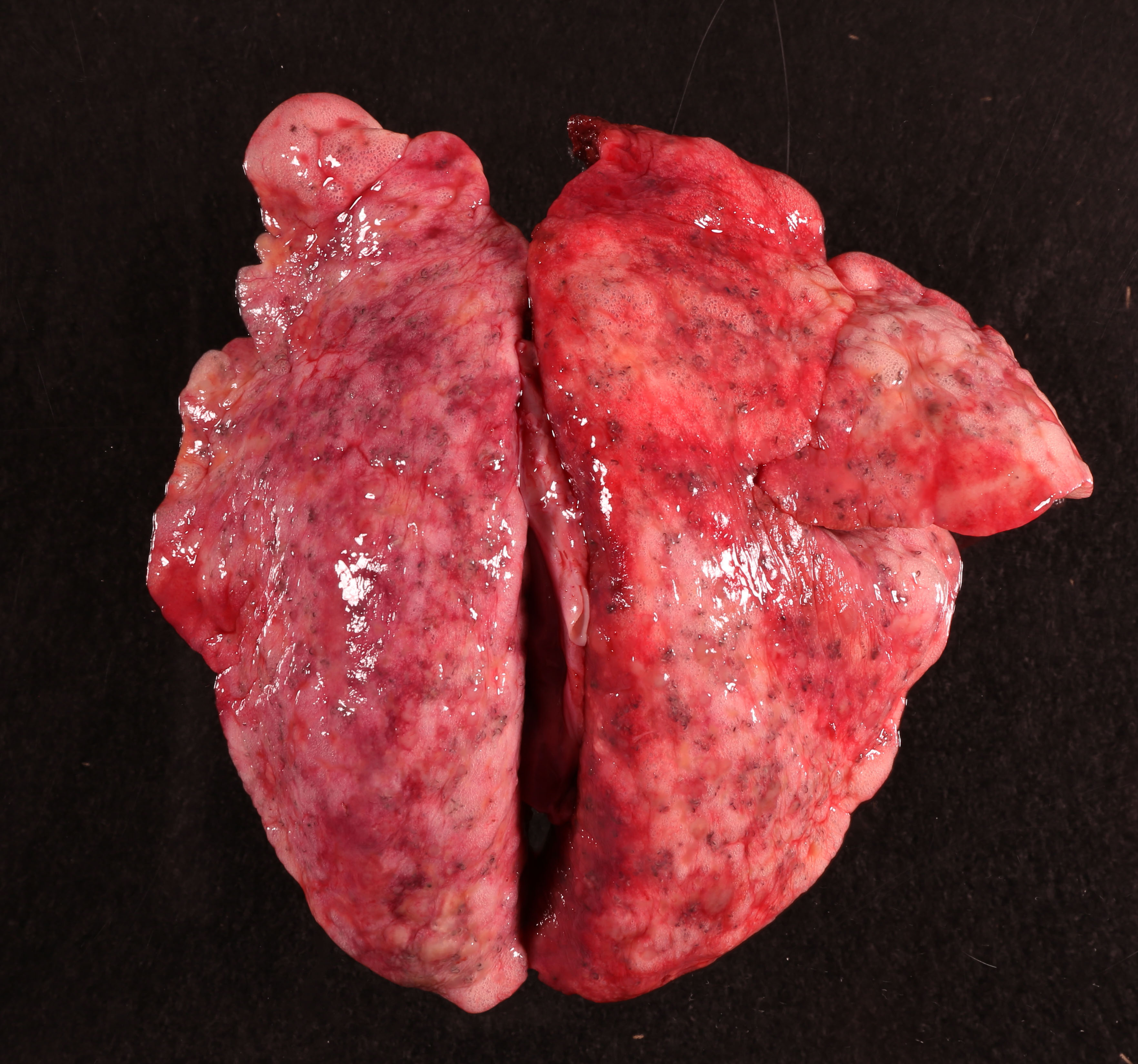
Diffusely, all the lung lobes were mottled pale pink, light yellow and white with myriad irregular, indistinct and coalescing nodules forming consolidated and semi-firm parenchyma and numerous tiny black and white, threadlike, curvilinear to serpentine foci randomly scattered throughout. The bronchi and bronchioles were filled with abundant yellow, thick, mucoid material. At the tip of the left cranial lung lobe is a small bulla.
Laboratory Results:
None.
Microscopic Description:
Lung: Nearly all the bronchi and bronchioles are distended (ectasia) and occluded by numerous larvae and a few adult nematodes intermixed with large numbers of degenerate and non-degenerate neutrophils, macrophages, lymphocytes, plasma cells, fewer eosinophils, sloughed epithelial cells, abundant cellular and pyknotic debris and mucus. The adult nematodes have a smooth cuticle with underlying hypodermis, lateral cords, meromyarian-platymyarian musculature. The pseudocoelom contains a digestive tract composed of large epithelial cells with a brush border and numerous intracytoplasmic brown granules, ovaries/testis and uterus harboring numerous embryonated ova and larvae. Larvae are approximately 90 um in length and 10 um in width with linear arrangment of nuclei. The bronchial and bronchiolar epithelium is extensively eroded, attenuated or hyperplastic. The submucosa is infiltrated by large numbers of lymphocytes, plasma cells, and macrophages, which also extend into the peri-bronchial tissues. In addition, bronchial smooth muscles are thickened (hypertrophy) and the peribronchial mucous glands are enlarged with abundant mucus production (hyperplasia). More than 60% of the alveoli, particularly adjacent to the airways, are filled and expanded by numerous adult nematodes and larvae as described above. The alveoli are also extensively effaced by multifocal to coalescing granulomas in which large numbers of macrophages, multinucleated giant cells and few other inflammatory cells are centered on nematode larvae. The granulomatous inflammation is vaguely intermixed and encircled by activated fibroblasts and collagen fibers that extend into the septa of adjacent alveoli. The alveoli subjacent to the granuloma are compressed and collapsed (atelectasis). Some of the remaining alveoli are distended and fused (emphysema). The pleura is intermittently lined by cuboid (reactive) mesothelial cells.
Contributor's Morphologic Diagnoses:
Lung: Pneumonia, granulomatous, multifocal to coalescing, chronic, severe with adult and larval metastrongyle sp., bronchiolar ectasia and occlusion, smooth muscle hypertrophy, pulmonary interstitial fibrosis, atelectasis, and emphysema, North American opossum (Didelphis virginiana), marsupial.
Contributor's Comment:
The Virginia opossum (Didelphis virginiana), commonly known as the North American opossum, is the only marsupial found north of Mexico. As a successful opportunist, they frequently inhabit urban areas due to the associated proximity to food sources. The opossum is a hardy creature that seems to adapt to heavy parasitic infections quite well.1,4 Interestingly, wild opossums are seldom reported to have rabies and to serve as a poor-quality host for ticks and pathogens (dilution hosts), diverting tick blood meals away from competent hosts.7 In addition, they are short-lived animals; few live longer than 2 years. Whether the short life span of the opossum correlates with parasitism is unknown. In this case, the opossum presented with severe emaciation, respiratory distress, and multiple traumatic injuries. At necropsy, poor body condition and heavy internal and external parasitism were identified. The stomach and duodenum are impacted by nematodes (Physaloptera turgida), which is very common in the opossum population as described1,6,7 and the small intestine was heavily parasitized by Mesoceastoides spp.1 However, comprehensive microscopic examination of multiple skeletal muscles and visceral organs from this opossum did not capture Besnoitia darlingi, which is commonly noted in opossum.
Didelphostrongylus hayesi belongs to the order Strongylida, superfamily Metastrongyloidea. With 48 to 79% of the prevalence rate, D. hayesi is one of the most common pulmonary nematodes in the opossum.1,7 D. hayesi has an indirect life cycle requiring terrestrial snails (Mesodon perigraptus or Triodopsis albolabris) as an intermediate host. After intermediate hosts are ingested, the larvae migrate from the alimentary tract to the lung, likely through the blood and lymphatic circulation and direct penetration. Subsequently, the third-stage larvae mature into the adult stage in the airways, particularly intrapulmonary bronchi. As presented in this case, numerous nematodes admixed with mucus and inflammatory cells occlude the bronchiolar lumina and alveolar spaces. Unlike other lungworms, D. hayesi are distinguished by ovoviviparity, in which the eggs are hatched within the uteri. The newly hatched larvae (L1) migrate up the trachea, are swallowed and are then shed in the feces.4 The pathology caused by D. hayesi can be mild to severe. In a recent study, 20 of 44 opossums trapped in the state of Colima, Mexico, carried D. hayesi in their airways, but none of them showed overt emaciation, suggesting that D. hayesi has little or no effect on the general health status.8 However, prominent gross pulmonary lesions were observed in 5 of 11 opossums with D. hayesi infection in another retrospective study.6 In this case, severe respiratory distress occurred as the result of airway obstruction, granulomatous pneumonia, and extensive fibrosis. The clinical deterioration was enhanced by comorbidities, including trauma-associated stress, malnutrition and gastric impaction due to heavy parasitism.
At necropsy, the gross changes in the lungs, including the diffuse consolidation of parenchyma with numerous granulomatous nodules, terminal emphysema, are impressive. Under the microscope, the consolidation of the lungs corresponds with bronchiolar and alveolar spaces filled by nematodes, inflammatory cells, and debris, in combination with granulomatous inflammation typically centered on larvae; adult worms evoked a relatively mild inflammatory response.2,6,8 Tiny black foci randomly scattered throughout the lungs, as grossly observed, correspond to the parasitic brown pigments within the epithelium of conspicuous digestive tracts of the nematodes.2 The inflammation is mainly composed of macrophages, multinucleated giant cells and lymphocytes, plasma cells. The eosinophilic component is minor, suggesting chronicity. In addition, the lungs failed to collapse due to the thickening of alveolar septa arising from interstitial fibrosis and infiltration of inflammatory cells. The emphysema and atelectasis are the consequence of verminous airway obstructions. Bronchiolar and alveolar ductular smooth muscle hyperplasia, recognized as a feature of D. hayesi in the lungs6, is noted in this case.
The morphological changes seen in this case are compatible with a late-stage lungworm infection in the opossum or other species, such as Aelurostrongylus abstrusus in cats.2,6,8 It is deemed that these parasites induce pulmonary injuries via direct mechanical irritation and/or their secretory products, which is exacerbated by enzyme and free radical released by inflammatory cells. Typical features include type II pneumocyte hyperplasia, alveolar bronchiolization, as well as hyperplasia and metaplasia of goblet cells in the airway, which are evidence of a response to the stimulation and damage of pneumocytes. Surfactant overproduction can also result in the accumulation of alveolar macrophages.8 In this case, there is severe granulomatous inflammation, fibrosis and smooth muscle hypertrophy that occasionally obscures these features. This suggests a relatively increased severity and chronicity. No evidence of concurrent bacterial coinfection or other parasite infection, such as Eucoleus aerophilus (formerly Capillaria aerophilus) or Besnoitia darlingi, was identified in this case.
Contributing Institution:
Department of Biomedical Sciences, Cummings School of Veterinary Medicine, Tufts University
200 Westboro Rd, N Grafton, MA 01536
Website: https://vetmed.tufts.edu/pathology-service/services/anatomic/
JPC Diagnosis:
Lung: Bronchopneumonia, lymphoplasma-cytic, diffuse, severe, with multifocal granulomas, marked smooth muscle hyperplasia, and metastrongyle adults, larvae, and eggs.
JPC Comment:
The contributor provides a concise summary Didelphostrongylus hayesi's lifecycle, prevalence, histologic features, and associated pulmonary lesions described in infected Virginia opossums (Didelphis virginiana).
D. hayesi is similar to many other metastrongyle lungworms in that adults are most commonly found in the bronchioles and bronchi, which may result in goblet cell metaplasia within the bronchiolar epitheium, a location not typically inhabited by goblet cells. As a result, chronic bronchiolitis may induce excessive mucous production, which in turn may form mucous plugs and obstruct lower airways. In addition, parasitized opposums may also exhibit hyperplasic bronchial glands, resulting in a high gland to wall thickness ratio, otherwise known as the Reid index. An increased Reid index is an indication of prolonged mucosal irritation and may be increased as the result of multiple conditions, such as allergies, inflammation, and parasitic bronchitis.8
Rarely, D. hayesi infection in opossums is associated with a lesion known as alveolar bronchiolarization. Also known as peribronchiolar metaplasia or "lambertosis" in human pathology, this phenomenon occurs as the result of an erratic remodeling process in which bronchiolar epithelial cells migrate to and repopulate alveoli following injury and fibrosis. Lambertosis is an eye-catching lesion given that it is composed of clusters of bronchiolar cells lining the alveolar basement membrane and is easily mistaken for pulmonary adenoma, as evidenced reports of "extensive adenomatoid proliferation of alveolar epithelium" in early reports describing opossums with verminous pneumonia.8
As noted by the contributor, the capillarid nematode Eucoleus aerophilus is also associated with verminous pneumonia in opossums, with dual infections commonly reported in the United States. Both D. hayesi and E. aerophilus are associated with granulomatous reactions, however, severe reactions are predominantly associated with the latter.8
Both adult D. hayesi and E. aerophilus nematodes may both be found in the bronchi. However, the former is also found in the bronchioles while the latter is also inhabits in the trachea of canids, felids, and some omnivorous animals such as opossums. Despite its wide host-range and zoonotic potential, little is known about E. aerophilus' life cycle although it is hypothesized animals become infected following the ingestion of an earthworm intermediate host. E. aerophilus infection is particularly common in wild foxes, with prevalence rates as high as 88% in Norway and 84% in the Pannonian and Fruska Gora Mountain regions of Serbia. In addition E. aerophilus is a parasite of economic importance as it is considered to be an agent of massive mortality in farmed silver foxes.5
E. aerophilus belongs to a group of nematodes known as aphasmids, which differ from other nematodes by lacking a pair of sensory papilla on the caudal aspect, known as phasmids. A characteristic feature of aphasmids in histologic sections is a structure composed of a row of esophageal gland cells (stichocytes), known as the stichosome, which surrounds the esophagus. In addition, aphasmids produce either embryonated or unembryonated eggs with bipolar plugs.3
During the conference, the moderator discussed a unique feature of D. hayesi amongst metastrongyles: its meromyarian-platymyarian musculature. This feature is in contrast to other metastrongyles, which have coelomyarian-polymyarian musculature. The reason for this variance is unclear, although the moderator mused this feature may represent a retained feature from an evolutionary divergence from nematodes with similar musculature, such as strongyles or trichostrongyles.
As noted in the contributor's description, the section includes multifocal areas of atelectasis and emphysema. Participants discussed these features and postulated the atelectasis likely occurred as the result of both bronchiolar obstruction with collapse of the downstream airways in addition to compression from adjacent granulomas. Furthermore, many remaining alveoli are emphysematous, which is consistent with the clinical history of dyspnea and likely increased inspiratory effort.
References:
1. Alden, KJ. Helminths of the oppossum, Didelphis virginiana, in southern Illinois, with a compilation of all helminths reported from this host in North America. J Helminthol Soc Wash. 1995;62(2):197-208.
2. Duncan RB Jr, Reinemeyer CR, Funk RS. Fatal lungworm infection in an opossum. J Wildl Dis. 1989;25(2):266-269.
3. Gardiner CH, Poynton SL. An Atlas of Metazoan Parasites in Animal Tissues, American Registry of Pathology. Washington, DC, 1999:40.
4. Jones, KD. Opossum nematodiasis: diagnosis and treatment of stomach, intestine, and lung nematodes in the virginia opossum (Didelphis virginiana). Journal of Exotic Pet Medicine. 2013;22(4):375-382.
5. Lalo?evi? V, Lalo?evi? D, Capo I, Simin V, Galfi A, Traversa D. High infection rate of zoonotic Eucoleus aerophilus infection in foxes from Serbia. Parasite. 2013;20:3.
6. Lamberski N, Reader JR, Cook LF, Johnson EM, Baker DG, Lowenstine LJ. A retrospective study of 11 cases of lungworm (Didelphostrongylus hayesi) infection in opossums (Didelphis virginiana). J Zoo Wildl Med. 2002;33(2):151-156.
7. Levi T, Keesing F, Holt RD, Barfield M, Ostfeld RS. Quantifying dilution and amplification in a community of hosts for tick-borne pathogens. Ecol Appl. 2016;26(2):484-498.
8. L?pez-Crespo RA, L?pez-Mayagoitia A, Ram?rez-Romero R, Mart?nez-Burnes J, Prado-Rebolledo OF, Garc?a-M?rquez LJ. Pulmonary lesions caused by the lungworm (Didelphostrongylus hayesi) in the opossum (Didelphis virginiana) in Colima, Mexico. J Zoo Wildl Med. 2017;48(2):404-412.