Wednesday Slide Conference, 2025-2026, Conference 1, Case 2
Signalment:
19-year-old, American miniature stallion.
History:
The horse presented with a 24-hour history of lethargy and possible neurologic signs. Circling to the left had been noted the day prior to admission, and the horse was frequently seen standing quietly with his head lowered. During physical examination, the horse was quiet, yet alert and responsive. A cranial nerve exam was within normal limits. quiet, yet alert and responsive. A cranial nerve exam was within normal limits. Blood work, including bile acids and ammonia levels, was unremarkable as were cervical spinal radiographs. The horse was treated with flunixin meglumine, Vitamin E, Marquis?, and intramuscular dexamethasone injections. Despite treatment for 4 days, the horse developed an intermittently absent menace of the left eye, a left head tilt, compulsive walking in the right direction, and a medial strabismus of the left eye. These signs progressed to full body muscle fasciculations, fixed dilated pupils, absent pupillary light reflex and menace bilaterally, head pressing, of the left eye. These signs progressed to full body muscle fasciculations, fixed dilated pupils, absent pupillary light reflex and menace bilaterally, head pressing, and a rising body temperature. Due to the horse?s worsening condition, humane euthanasia was elected.
Gross Pathology:
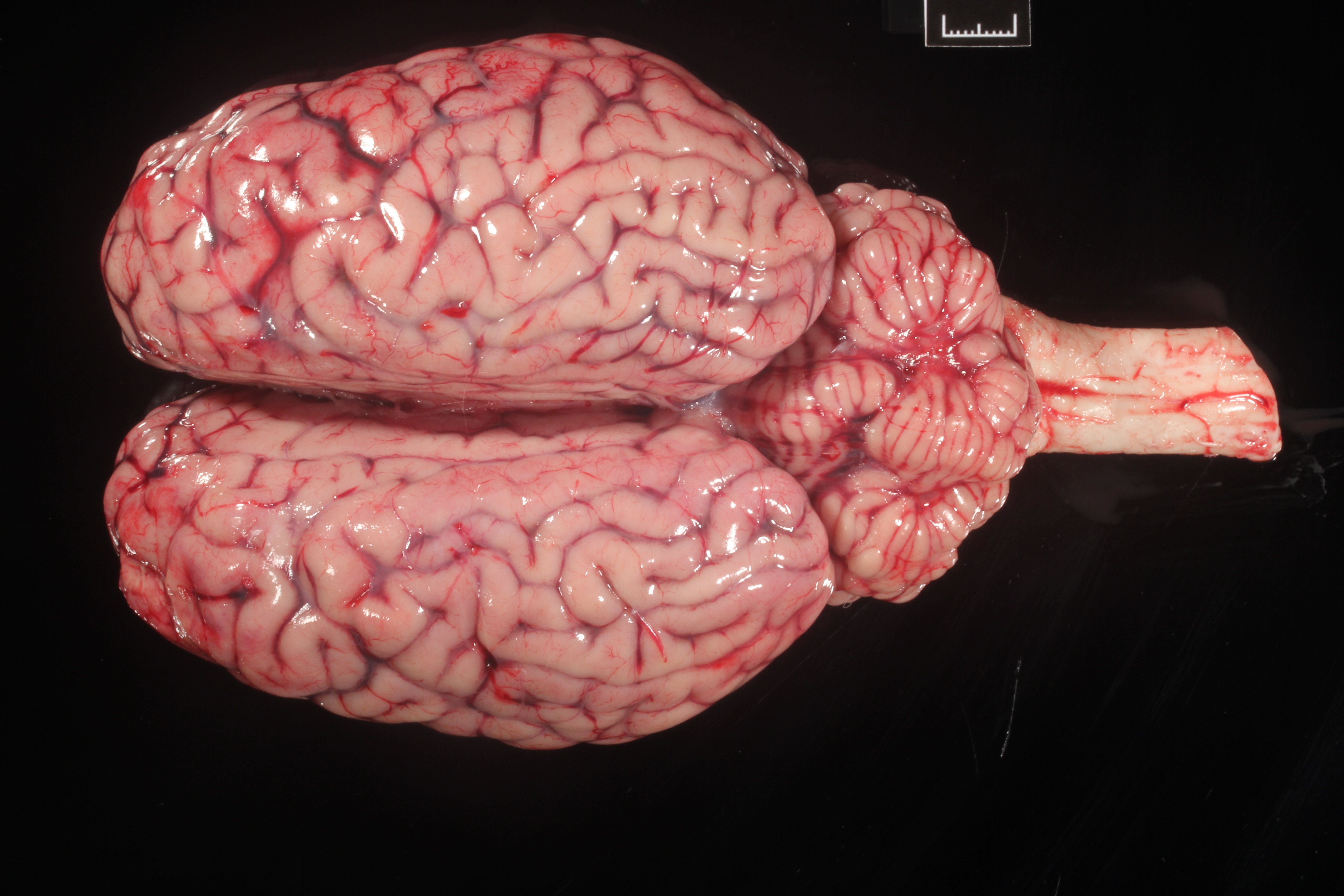
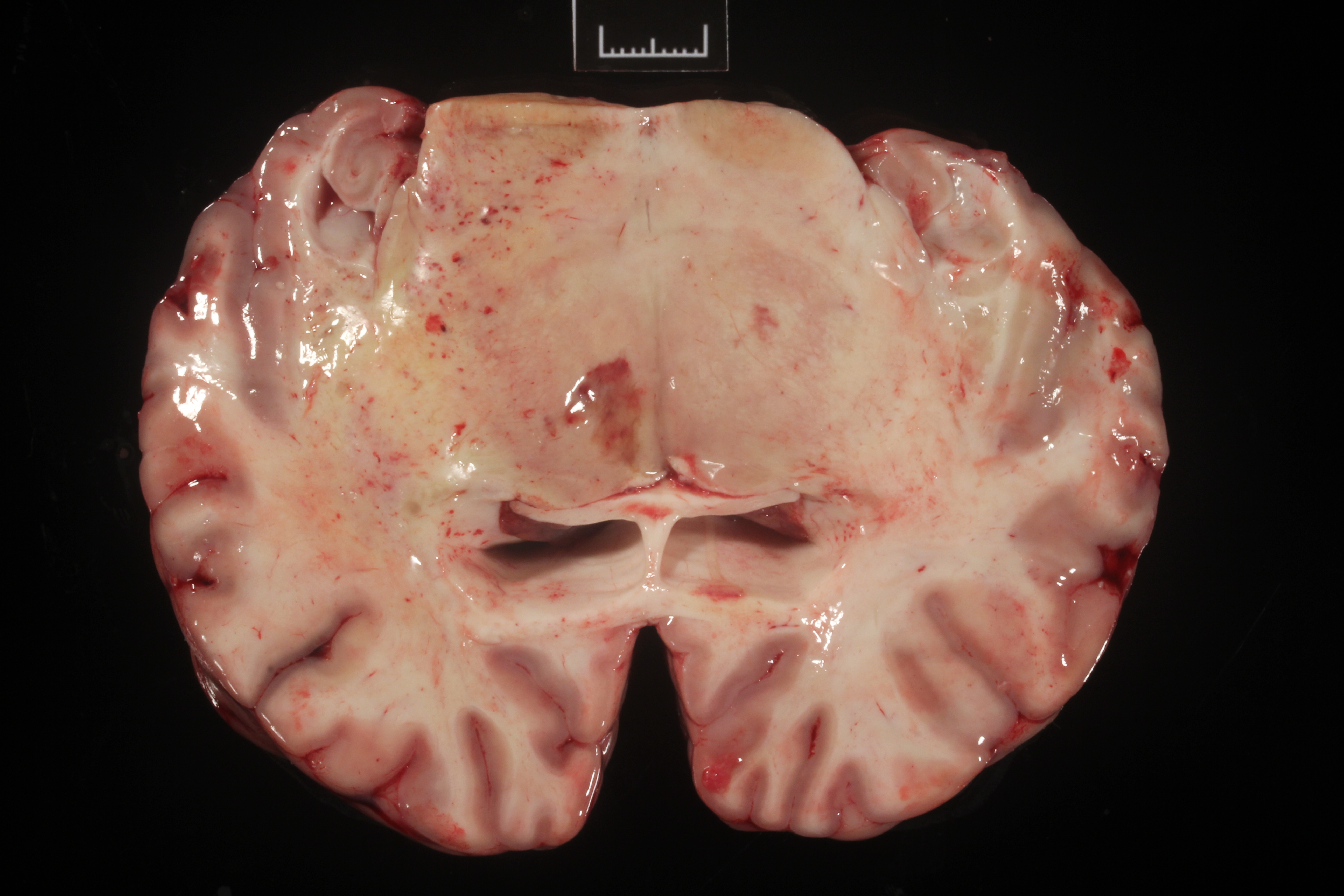
The right frontal lobe was slightly enlarged (asymmetry) (Fig.1). On cut surface, the white matter was mildy expanded and yellow (edema). A 1.1x0.5 cm, soft, red-brown area was within the right thalamus (malacia).
Laboratory Results:
Rabies fluorescent antibody testing: negative; Eastern Equine Encephalitis virus RT-PCR: negative; Western Equine Encephalitis virus RT-PCR: negative; West Nile virus RT-PCR: negative; St. Louis Encephalitis virus RT-PCR: negative.
(
https://tvmdl.tamu.edu/tests/panfungal-pcr/)
Panfungal PCR targeting the internal transcribed spacer (ITS) region and agarose gel electrophoresis yielded 2 bands of DNA at approximately 350bp and 450bp. DNA was purified from the gel and sequenced. The resulting 119bp and 295bp sequences were analyzed with the NCBI BLAST database. The sequence matched
Chaetomium strumarium and
Malassezia restricta with 100% identity.
Microscopic Description:
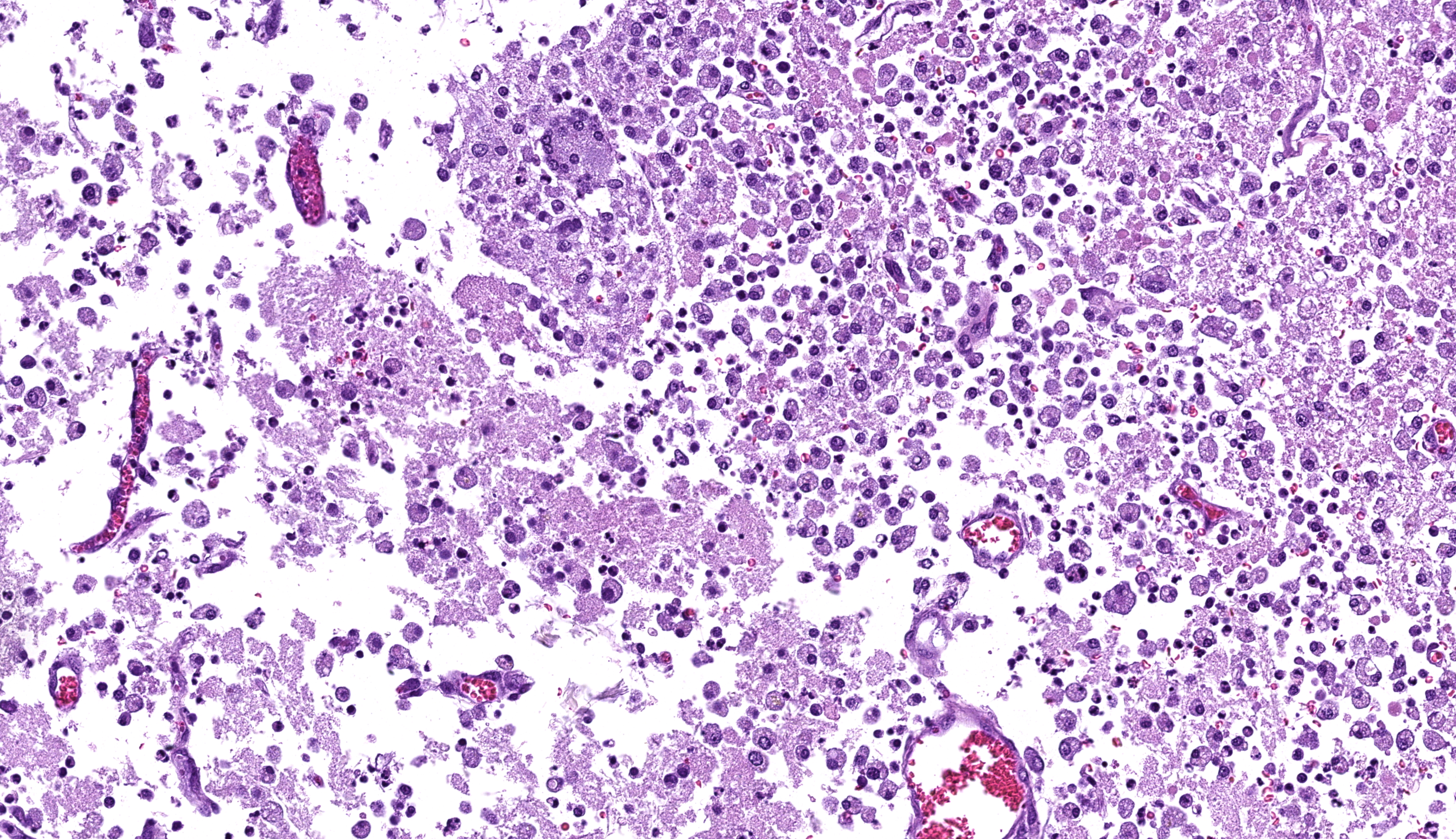
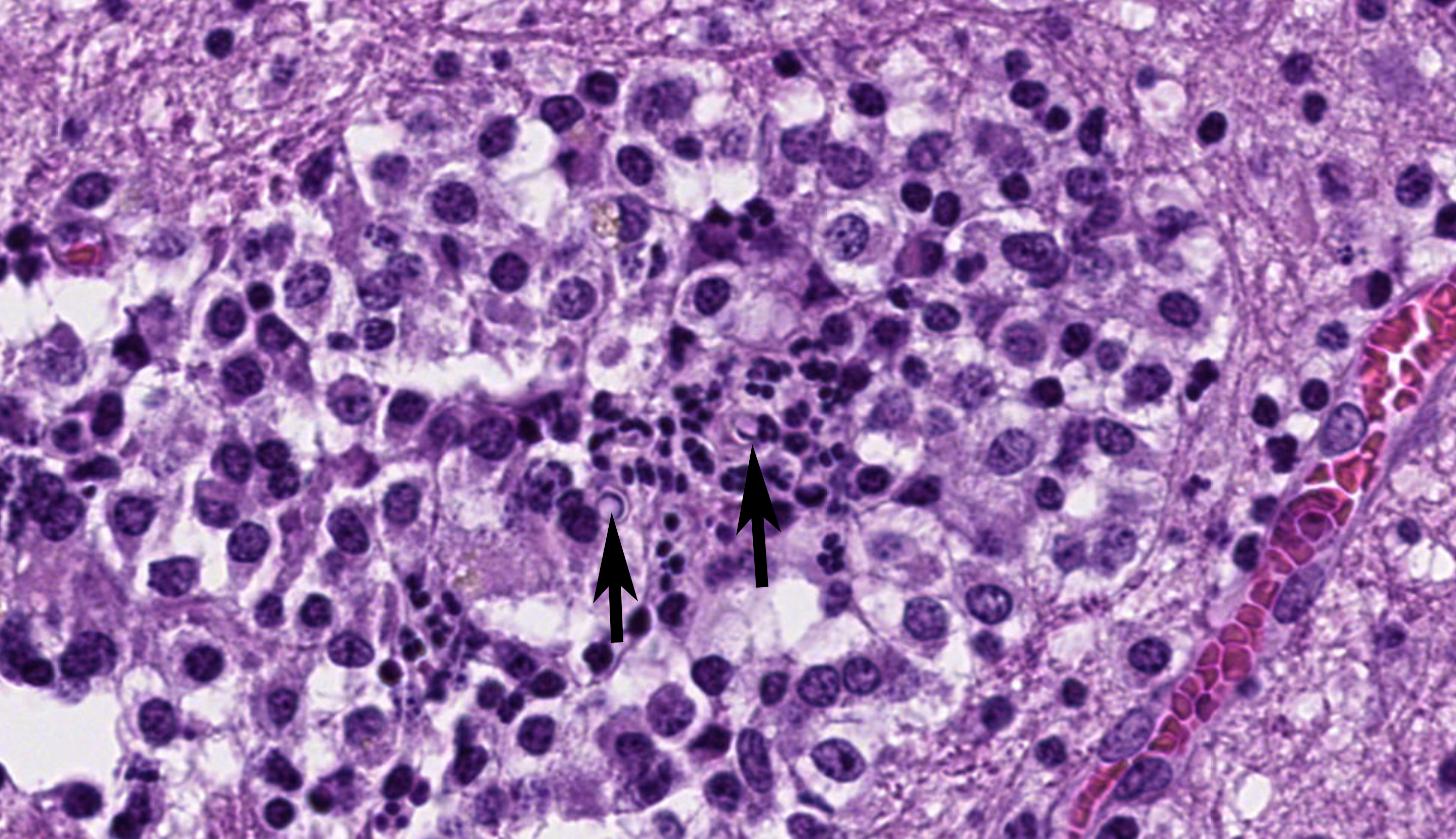
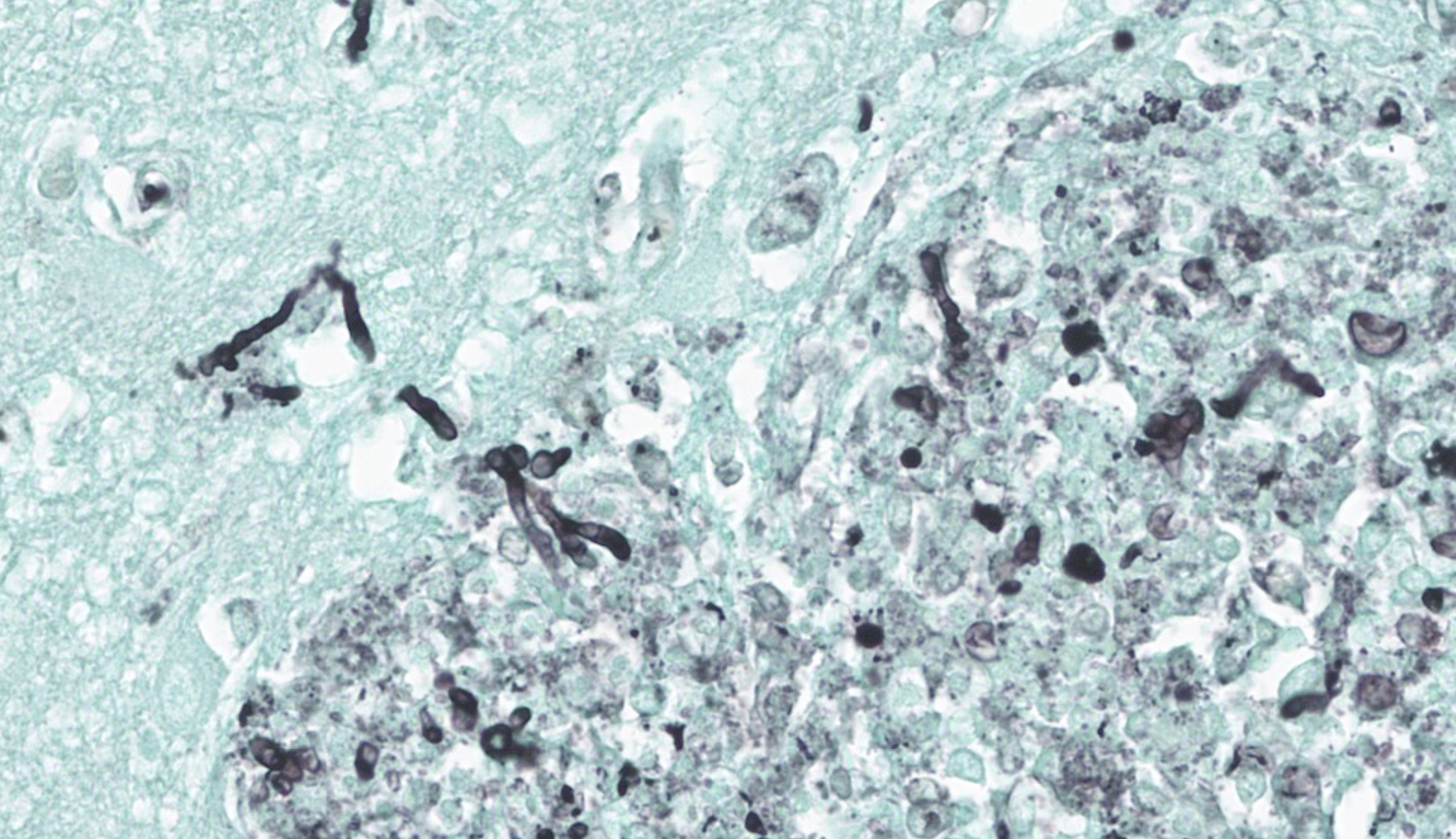
Cerebrum: Multifocally and randomly infiltrating the neuroparenchyma are numerous distinct accumulations of inflammatory cells composed of epithelioid macrophages and neutrophils with fewer lymphocytes, multinucleated giant cells (Langhans and foreign body type), and plasma cells. Inflammatory cells also frequently and moderately expand Virchow-Robins? spaces. Admixed extracellularly with inflammatory cells and phagocytosed within macrophages and multinucleated giant cells are sections of golden-brown, 7-10um pigmented fungal hyphae characterized by frequent septation, dichotomous branching, and terminal bulbous swellings. The section also exhibits multifocal areas of cavitation with loss of neuroparenchyma and replacement by numerous foamy macrophages (gitter cells) accompanied by increased numbers of astrocytes and microglia (gliosis) with increased clear space (edema) and areas of hemorrhage. Neurons in these areas are multifocally shrunken and hypereosinophilic with pyknotic nuclei (necrosis) and axons are frequently swollen and hypereosinophilic and surrounded by dilated myelin sheaths (spheroids).
Contributor's Morphologic Diagnoses:
Cerebrum: Marked, multifocal to coalescing, chronic, pyogranulomatous encephalitis with extracellular and intrahistiocytic fungal hyphae.
Contributor's Comment:
Histologic evaluation identified fungal encephalitis as the cause of the horse?s neurologic signs with PCR results identifying the dematiaceous fungus,
Chaetomium sp., as the cause. Meningoencephalitis associated with dematiaceous fungi has been previously described in horses
10.
The term "dematiaceous" has been used to describe fungi that are olivaceous, dark brown, or black due to the presence of melanin or melanin-like pigment within the cellular walls
13. It has been suggested that the term ?dematiaceous? is not appropriate given its etymologic derivation from the Greek ?deme,? meaning ?bundle.? The term ?melanized? has been used more recently to describe pigmented fungi
11. Melanized fungi are responsible for causing a wide range of diseases including chromoblastomycosis and phaeohyphomycosis. Chromoblastomycosis is a chronic infection of the skin and subcutaneous tissues, characterized by muriform bodies or sclerotic bodies, and typically caused by
Fonsecaea pedrosoi, Fonsecaea compacta, Phialophora verrucosa, or
Cladosporium carrionii13.
The name "phaeohyphomycosis" is derived from the Greek word ?phaeo? meaning ?dusky? or ?grey.? In phaeohyphomycosis, the tissue morphology of the fungus is predominantly composed of hyphae (mycelial). Although the agents of phaeohyphomycosis may exhibit a dark coloration in culture and the gross appearance of these lesions may be partially pigmented, these organisms appear non-pigmented in histologic sections stained with using H&E. In such cases, Fontana-Masson may be helpful in identifying the pigmented hyphae, while Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) usually obscure the pigment
13.
In humans, phaeohyphomycosis causes opportunistic infections in the cornea, sinus, skin, and lungs of immunocompromised patients, with the exception of central nervous system infections, which tend to affect immunocompetent individuals. Genera involved with phaeohyphomycosis in humans include:
Alternaria, Bipolaris, Chaetomium, Cladophialophora, Curvularia, Exophiala, Exserohilum, Lasiodiplodia, Lecythophora, Ochroconis, Phaeoacremonium Pseudallescheria, Rhinocladiella, and
Scedosporium, among others
13.
Fungi of the members of the orders Chaetothyriales, Pleosporales, Ochroconiales, and Capnodiales are involved in phaeohyphomycosis in a wide range of cold-blooded vertebrates, such as crustaceans, captive and farmed fish, amphibians, and aquarium animals. Phaeohyphomycosis is less prevalent or less recognized in warm-blooded animals
12. Cats appear to be more susceptible with case reports of this infection documenting nasal, renal, cutaneous, subcutaneous, ocular, cerebellar and systemic involvement
12,14. Members of the family Chaetomiaceae are ubiquitous fungi which reside in soil enriched with manure or cellulosis-decaying materials
1,2. A certain prevalence of chaetomium-like species was noted in desert soil subjected to conditions of dryness and extremely variable temperatures
2.
Chaetomium atrobrunneum,
C. perlucidum, and
C. strumarium are neurotropic species causing serious and life-threatening infections in people
1,3.
Mycotic encephalitis is rare in horses. Reported cases include aspergillosis
4, one of which presented with concurrent Mucor sp. infection, and cryptococcosis. Recently, phaeohyphomycosis within the Chaetomiaceae family, including
Acrophialophora fusispora,
Acrophialophora levis, and
Chaetomium strumarium has been described as novel causes of equine neurotropic mycosis. Affected animals ranged from 8 to 22 years of age. The most common central nervous system location was the cerebrum
10.
Panfungal PCR on formalin-fixed paraffin-embedded (FFPE) tissues targeting the ribosomal RNA large subunit coding region and the noncoding internal transcribed spacer-2 region is a useful technique when fresh tissue is not available for culture. Although pigmented fungi may appear to be a straightforward identification on H&E sections, variability exists in the amount of melanin produced by a genotypic variant, and thus these fungi could be confused with other hyaline hyphae. Additionally, pigmented fungi cannot be identified to the genus level using histologic evaluation alone
5. In this case, the amplified
Malassezia restricta sequence is a common contaminant in paraffin blocks and unrelated to the cause of the fungal infection in this case. Panfungal PCR and sequencing results should always be correlated with the assessment of fungal morphology.
Contributing Institution:
Department of Veterinary Pathobiology, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University
JPC Diagnoses:
Cerebrum: Encephalitis, necrotizing and pyogranulomatous, focally extensive, severe, with vasculitis and rare pigmented fungal hyphae.
JPC Comment:
This is a excellent case of a fungal encephalitis in a horse caused by a unique mycotic pathogen. The contributor gives a thorough overview of this condition and other relevant fungi in their comment. While it is well-established that the brain and retina of horses, as well as the nervous system and skin of cats, are frequent targets of dematiaceous fungi in veterinary species, this particular pathogen is rarely isolated from infections in animals.
This conference case stimulated discussion on how, despite the challenge of finding the hyphae on the H&E slide, the type of inflammation (pyogranulomatous) should make the pathologist suspicious for particular types of pathogens and stimulate careful search in affected areas for those agents. A GMS stain readily revealed the fungal hyphae within the affected neuropil and was shown to conference participants following the H&E description. A great review of microglia and their roles followed case presentation, including the differences between resting microglia, gitter cells, multinucleated giant cells, and ?rod? cells. A brief review of the necessary fungal features to mention in a slide description was covered and included: size, branching (acute vs right angle, dichotomous vs irregular, and frequency), parallel vs. non-parallel walls, septation, pigmented or non-pigmented, and mention of whether the fungus is angioinvasive or not. One conference participant also made an excellent point that, after others questioned the pigmentation of the fungi outside of that seen in histiocytes, that pigmented fungi are classified as such based on their appearance on a growth plate and not on an H&E slide.
A closer look at
Chaetomium sp fungi helps elucidate how they cause illness.
Chaetomium sp are well-documented neurotropic fungal pathogens of humans, but until recently, were not associated with disease in veterinary species11. "Chaeto-" comes from the Greek word "chait?", meaning "long hair" in reference to the filamentous setae covering the fruiting bodies of these fungi. These setae are considered one of the defining morphologic features of this fungal genus, and differing setae characteristics within the genus can assist in speciation.
These opportunistic fungi, when in an environment that enables their growth, produce a variety of mycotoxins, including several types of chaetoglobosins. Chaetoglobosins are a type of cytochalasin fungal metabolite that binds to and prevents the polymerization of actin filaments in animal cells, inhibiting cellular movement, changing cellular morphology, and hindering mitosis, ultimately resulting in apoptosis of the affected cell
5. Chaetoglobosin A, in particular, is highly toxic to mammalian cells and, even at low doses, has been demonstrated to be acutely fatal in laboratory rats and mice
9. Other produced toxins, such as chaetoviridins, have antimicrobial and antifungal properties against other fungi, enabling the
Chaetomium sp to thrive while inhibiting the growth of competitors
4.
Chaetoviridins have also been shown to inhibit tumor development in carcinogenic mice models via targeted cytotoxic effects. As such, chaetoglobosins and chaetoviridins are currently being studied for their use as potential antimicrobial and chemotherapeutic agents6.
Chaetomiaciae sp are yet another excellent example of the natural world enabling biochemists and pharmacologists to walk the fine line between therapeutic dosage and toxicity.
References:
- Abbott SP, Sigler L, McAleer R, et al. Fatal cerebral mycoses caused by the ascomycete Chaetomium strumarium. J Clin Microbiol. 1995;33(10): 2692?2698.
- Ahmed SA, Ziauddin K, Wang X, et al. Chaetomium-like fungi causing opportunistic infections in humans: a possible role for extremotolerance. Fungal Diversity. 2016:76:11?26.
- Barron MA, Sutton DA, Veve R, et al. Invasive mycotic infections caused by Chaetomium perlucidum, a new agent of cerebral phaeohyphomycosis. J Clin Microbiol. 2003;41(11):5302?5307.
- Elkhateeb WA, Kolaibe AG, Elnahas MO, Daba GM. Highlights on Chaetomium morphology, secondary metabolites and biological activates. J Pharmaceutics and Pharmacology Research. 2021;4(1).
- Fogle MR, Douglas DR, Jumper CA, Straus DC. Growth and mycotoxin
production by Chaetomium globosum is favored in a neutral pH. Int J Mol Sci. 2008;9(12):2357-2365.
- Goda MS, El-Kattan N, Abdel-Azeem MA, Allam KAM, Badr JM, Nassar NA, Almalki AJ, Alharbi M, Elhady SS, Eltamany EE. Antimicrobial Potential of Different Isolates of Chaetomium globosum Combined with Liquid Chromatography Tandem Mass Spectrometry Chemical Profiling. Biomolecules. 2023 Nov 21;13(12):1683.
- Hunter B, Nation PN. Mycotic encephalitis, sinus osteomyelitis, and guttural pouch mycosis in a 3-year-old Arabian colt. Can Vet J. 2011;52(12):1339?1341.
- Meason-Smith C, Edwards EE, Older CE, et al. Panfungal polymerase chain reaction for identification of fungal pathogens in formalin-fixed animal tissues. Vet Pathol. 2017;54(5):640?648.
- Ohtsubo K, Saito M, Sekita S, Yoshihira K, Natori S. Acute toxic effects of chaetoglobosin A, a new cytochalasan compound produced by Chaetomium globosum, on mice and rats. Jpn J Exp Med. 1978 Apr;48(2):105-10.
- Plumlee P, Meason-Smith C, Dieterly, et al. Chaetomiaceae Fungi, Novel Pathogens of Equine Neurotropic Phaeohyphomycosis. Vet Pathol. 2017;54(5):813-819.
- Revankar SG, Sutton DA. Melanized Fungi in Human Disease. Clin Microbiol Rev. 2010:884-928.
- Seyedmousavi S, Guillot J, de Hoog GS. Phaeohyphomycoses, emerging opportunistic diseases in animals. Clin Microbiol Rev. 2013;26(1):19?35.
- Schmitt BH, Pritt B. Dematiaceous Fungal Infections. In: Procop GW, Pritt BS, ed. Pathology of Infectious Diseases. 1st ed. Saunder; 2014: 516-530.
- Velazquez-Jimenez Y, Hernandez-Castro R, Romero-Romero R. Feline phaeohyphomycotic cerebellitis caused by Cladosporium cladosporioides-complex: case report and review of literature. J Comp Path. 2019:170:78-85.