Wednesday Slide Conference, 2025-2026, Conference 8, Case 4
Signalment:
10 week old male mixed-breed puppy (Canis familiaris, dog)History:
Gross Pathology: There was mild generalized icterus of mucous membranes and soft tissues. The spleen was enlarged and meaty in texture on cut section. The liver was pale. The renal cortices were dark red-grey, and the urinary bladder contained red-tinged urine.Laboratory Results:
Fresh-frozen lung was submitted to the Vector-Borne Disease Lab at North Carolina State University for PCR analysis for Babesia, Anaplasma, Bartonella, Ehrlichia, Rickettsia, and Hemotropic Mycoplasma spp. PCR was positive for Babesia vogeli.Microscopic Description:
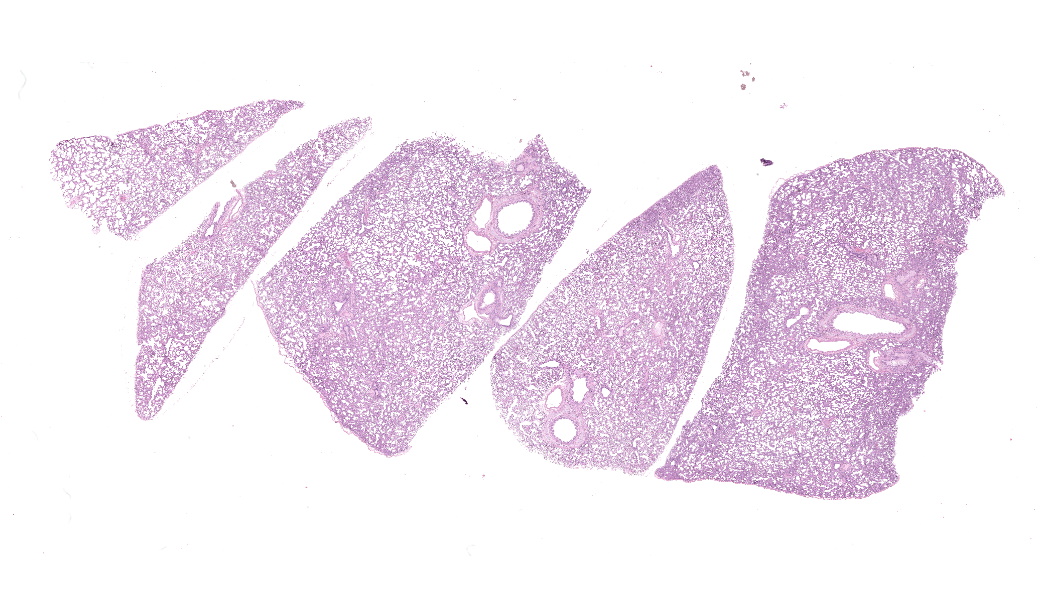
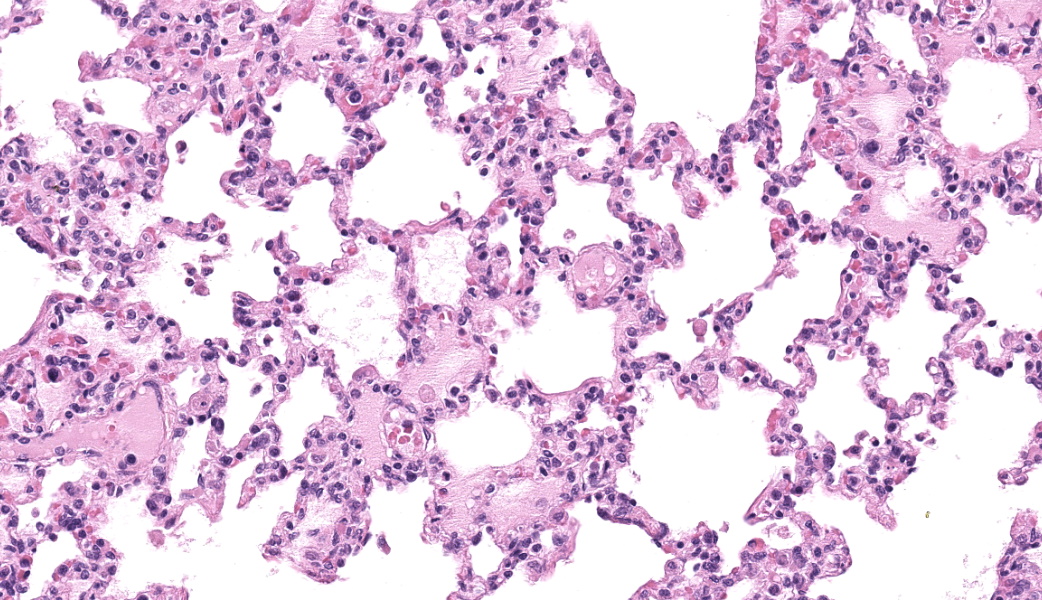
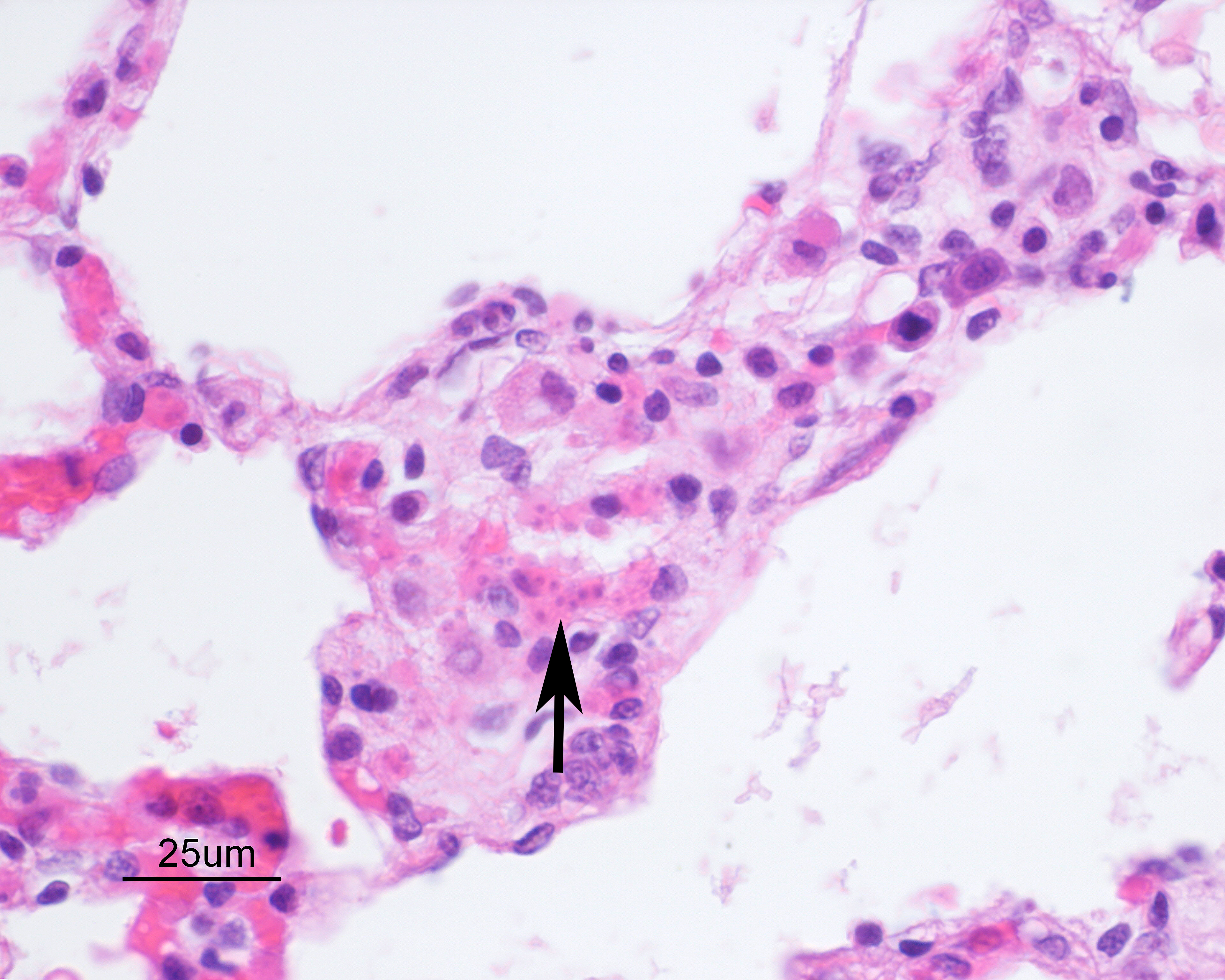
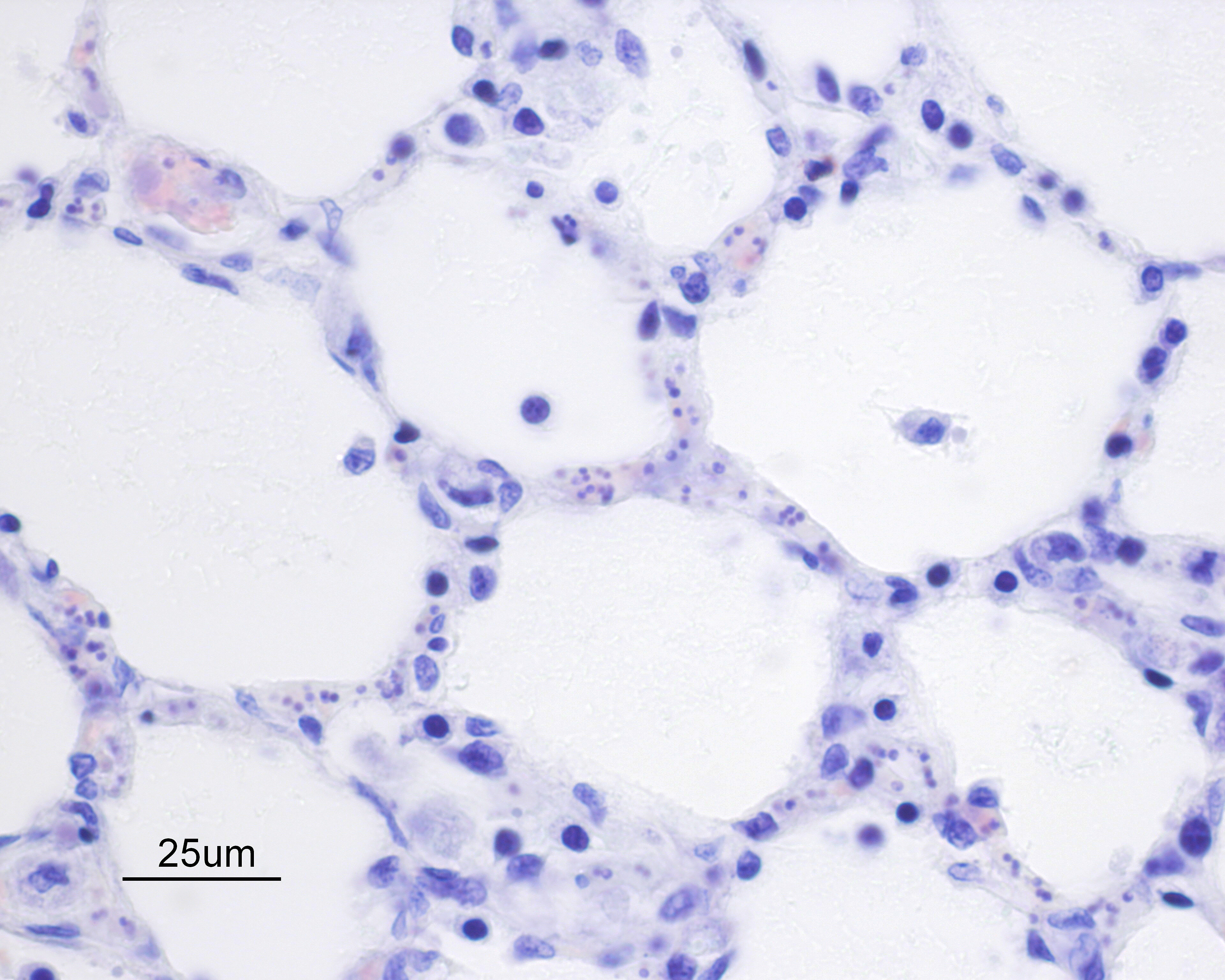
Alveolar septa are mildly thickened and hypercellular, and contain increased numbers of macrophages, rare neutrophils, and small quantities of fibrin (occasionally suspicious for capillary fibrin thrombi). Alveolar lumens contain increased numbers of free-floating macrophages, very rare neutrophils, and protein-rich fluid. Most intravascular erythrocytes contain small (1-3 micron) intracytoplasmic basophilic bodies, most visible in alveolar capillaries around the periphery of the histologic sections. Giemsa stains accentuate these small round faintly vacuolated organisms within erythrocytes.Contributor's Morphologic Diagnoses:
Generalized histiocytic and neutrophilic alveolitis with multifocal capillary thrombosis and intravascular, intraerythrocytic parasites (Babesia vogeli)Contributor's Comment:
Babesia spp. are tick-transmitted, apicomplexan, hemoprotozoan parasites. They are closely related to Cytauxzoon spp. and Theileria spp., and these three genera are termed piroplasmids. Babesia spp. was initially identified as a hemoparasite of cattle in Romania in 1888, causing hemoglobinuria, a syndrome termed “Red Water Fever”. Soon after, similar organisms were identified in cattle in Texas, and today Babesia spp. are recognized world-wide as tick-borne parasites of wild and domestic ruminants, equids, pigs, dogs, and cats8. Internationally, these parasites are of economic concern due to their impact on livestock health and productivity. Humans are also susceptible to Babesia spp., including B. microti, B. venatorum, B. divergens, B. duncani, B. divergens, and other unnamed Babesia spp., and human transmission has also been documented horizontally, or via blood transfusion7.Dogs have been reportedly infected with B. vogeli, B. conradae, B. gibsoni, B. vitali, B. rossi, B. canis, and unspeciated Babesia spp7. Original studies classified canine Babesia spp. based on morphology, with larger (3-5 micron) forms named B. canis, and smaller (1-3 micron) forms named B. gibsoni. However, it was suspected based on differences in pathogenicity that B. canis was heterogeneous, and with the advent of molecular diagnostic techniques, at least four small and four large Babesia parasites have been identified. B. canis was reclassified into three separate species (B. canis, B. vogeli, B. rossi)5. Babesia vogeli is transmitted by the Brown Dog tick (Rhipicephalus sanguineus) and has a wide endemic range throughout tropical and subtropical regions of the world, occasionally extending into cooler latitudes5.
In dogs, clinical babesiosis varies from mild to severe forms, manifesting as hemolysis and secondary systemic hypoxic and inflammatory organ injury, organ dysfunction, shock, and death1. The severity of clinical disease varies with a number of factors, including parasite species, and host age, immune status, and the presence of concurrent infection. The least pathogenic species recognized is B. vogeli, the most virulent is B. rossi. While B. vogeli more frequently subclinical, it is notably more pathogenic in puppies younger than 3-4 months5. A number of mechanisms have been implicated for the parasite-mediate hemolysis, including direct parasite-mediated injury, osmotic fragility of infected erythrocytes, oxidative stress, and secondary immune-mediated cell membrane injury, resulting in mixed intravascular and extravascular hemolysis5.
Common hematologic abnormalities include anemia, thrombocytopenia, and variable leukopenia or leukocytosis. Serum biochemical abnormalities include increased activity of aspartate aminotransferase and alanine aminotransferase, hyperbilirubinemia, hypoalbuminemia, and variable electrolyte and acid-base imbalances. Eichenberger et al. reported that the prognosis of clinically ill dogs infected with Babesia canis was negatively impacted by hyperlactatemia, leukopenia, hyperphosphatemia, hypertriglyceridemia, and hypoproteinemia1. Similarly, prognosis for B. rossi infection has been found to be impacted by hyperlactatemia, hypoglycemia, clinically compromised circulation/consumptive coagulopathy, and increased serum cortisol1.
Preliminary diagnosis of acute clinical Babesiosis typically requires evaluation of blood smears, usually using Giemsa or Wright’s stain. The likelihood of diagnosis, particularly with large Babesia spp., may be improved through specific evaluation of blood smears from capillary beds (ear tips, toenail) or evaluation of cells beneath the buffy coat of a hematocrit tube. In chronic cases, parasitemia may be minor and intermittent, making blood smear diagnosis more challenging5. PCR, immunofluorescence, and ELISA testing have also improved the rate of diagnosis of babesiosis, and PCR testing has become the principle means of diagnosis and species determination5. However, false negative results may be seen in cases of chronic babesiosis because of limitations similar those of blood smear diagnosis, and detection of chronic/carrier animals often requires repeated testing and use of complimentary testing methods2,6.
Importation of domestic dogs to Canada from other countries is becoming more common, though this is not well-tracked and reliable statistics are not available. This pattern of importation provides a risk of infectious diseases being imported along with them, and because some of these pathogens depend on environments or vectors not found in Canada, recognition may be delayed. However, because of the presence of competent tick vectors in some regions of Canada, the importation of dogs with babesiosis may provide a risk for disease transmission to local dog populations. Hemotropic parasites such as Babesia, Anaplasma, Mycoplasma, Bartonella, Ehrlichia, and Rickettsia spp. should be considered as possibly subclinical infections in imported dogs, and blood testing (including review of blood smears) prior to importation of dogs from endemic areas is recommended to reduce the risk of establishing infection within Canadian tick populations.
Contributing Institution:
Animal Health Laboratory, University of Guelph, Guelph, Ontario, Canada http://www.guelphlabservices.com/AHL/JPC Diagnoses:
Lung: Pneumonia, interstitial, histiocytic and neutrophilic, subacute, diffuse, moderate, with intraerythrocytic piroplasms.JPC Comment:
MAJ Fiddes chose this case specifically because it was going to be a diagnostic challenge. The intraerythrocytic piroplasms of Babesia sp. are quite difficult to see until you find one and got an eye for them within erythrocytes; then, they were much easier to spot. A few participants were successful in finding these small parasite. Along with discussion on the basics and life cycle of Babesia sp., which the contributor’s comment beautifully covers, conference goers participated in a matching game of other boards-worthy infectious hemotropic agents of note. These agents and their matching facts included Anaplasma sp., which may infect platelets, Theileria sp., which infect leukocytes and are another genus of piroplasm, Mycoplasma sp., which tend to reside in an epicellular location, and Cytauxzoon felis, yet another piroplasmid and one that has a bobcat reservoir host.Babesia sp. are apicomplexan piroplasmid protozoans that infect erythrocytes in numerous species, ultimately lysing infected red blood cells (RBCs) and resulting in intravascular hemolysis within the host. As mentioned, by the contributor, there are several species of Babesia that infect dogs, and there is a wide range of severity in the clinical manifestations amongst these. The focus here will primarily be on Babesia canis, which infects dogs, and Babesia bovis, the species responsible for most Babesia infections in cattle and has a substantial amount of research behind it.
Most of the virulence factors that Babesia is equipped with are responsible for survival and pathogenicity. Like other apicomplexans, Babesia’s virulence factors are generally secreted to the host cell surface, where they structurally remodel and biochemically modify the infected RBC’s ability to interact with specific host proteins in an effort to promote their own survivability. The most notable of these numerous virulence factors is the Virulence, Evasion, and Secretion-associated (VESA) proteins. VESA is responsible for inhibiting the binding of infected erythrocytes to endothelial cells, as well as reversing the binding of already-adhered erythrocytes.3,7 This ensures that, when the piroplasms have reproduced via binary fission and are ready to lyse the infected red cell, that they will remain within the vasculature to be picked up by a tick vector.
VESA proteins cluster on the surface of erythrocyte within ridges that are structurally induced by Babesia.3 The number of ridges increases as the piroplasmids grow and multiply within the erythrocyte.3 With VESA being expressed on the surface of infected red cells, the immune system should be able to locate and target those cells for antibody-mediated immune response, right? Yes, but no. Babesia bovis has demonstrated that it can express antigenic variants of VESA through epigenetic alterations and/or genomic recombination at the locus of active transcription that is responsible for the production of VESA proteins.3,7 This alters the VESA protein enough antigenically to become a constant moving target for immune cells, contributing to the chronic and recurrent nature of babesiosis.3 It is yet another prime example of the evolutionary arms race between infectious agents and immune cells, and it is currently speculated that many other virulent Babesia sp can perform the same epigenetic alterations to their VESA antigens.
There was some discussion on the presence of megakaryocytes within the affected lung sections in this case. Megakaryocytes can be present normally in low numbers in the lungs or can be seen in increased numbers secondary to an immune response. Megakaryocytes normally function to produce platelets, and a single megakaryocyte can make them by the thousands. In an immune response, though, megakaryocytes display an impressive range of functions related to pathogen recognition, antigen presentation, and inflammatory modulation.4 Lung megakaryocytes, specifically, express higher levels of pathogen recognition receptors (PRRs), specifically, toll-like receptors (TLRs) and C-type lectin-like receptor (CLEC). Lung megakaryocytes also sense and uptake pathogens, can release a gamut of inflammatory cytokines, function as antigen-presenting cells, and participate in immune responses against pathogen invasion.4 Because of their wide range of functions and key physiologic roles, megakaryocytes are sometimes referred to as an “omnipotent” cell type.4 In this case, it was the opinion of conference participants that the megakaryocytes were likely present due to the inflammation and protozoal infection due to the higher-than-expected number seen within section.
References:
- Eichenberger RM, Riond B, Willi B, Hofmann-Lehman R, Deplazes P. Prognostic markers in acute Babesia canis infections. J Vet Intern Med. 2016;30:174-182.
- Goo Y, Jia H, Aboge GO, et al.. Babesia gibsoni: Serodiagnosis of infection in dogs by an enzyme-linked immunosorbent assay with recombinant BgTRAP. Exp Parasitol. 2008;118:555-560.
- Hakimi H, Yamagishi J, Sakaguchi M, Fathi A, Lee JS, Verocai GG, Kawazu SI, Asada M. ves1α genes expression is the major determinant of Babesia bovis-infected erythrocytes cytoadhesion to endothelial cells. PLoS Pathog. 2025;21(4):e1012583.
- Hua T, Yao F, Wang H, Liu W, Zhu X, Yao Y. Megakaryocyte in sepsis: the trinity of coagulation, inflammation and immunity. Crit Care. 2024;28(1):442.
- Irwin PJ. Canine babesiosis: from molecular taxonomy to control. Parasit Vectors. 2009;2(suppl 1):1-9.
- Jefferies R, Ryan UM, Jardine J, et al. Babesia gibsoni: Detection during experimental infections and after combined atovaquone and azithromycin therapy. Exp Parasitol. 2007;117:115-123.
- Schetters T. Mechanisms Involved in the Persistence of Babesia canisInfection in Dogs. Pathogens. 2019;8(3):94.
- Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: A world emerging. Genet Evol. 2012;12:1788-1809.
- Uilenberg G. Babesia – A historical perspective. Vet Parasitol. 2006;138:3-10.