CASE 4: K18-1448 (4127786-00)
Signalment:
16 years old, gelding, Thoroughbred, Equus caballus, equine
History:
Intermittent fever for five weeks, which improved with administration of tetracycline, but then fever returned, and the horse developed a cough. Rhodococcus equi pneumonia was confirmed, and results of polymerase chain reaction (PCR) testing for Equid herpesvirus-2 and EHV-5 on tracheal wash fluid were positive. Concurrent equine multinodular pulmonary fibrosis was suspected.
Gross Pathology:
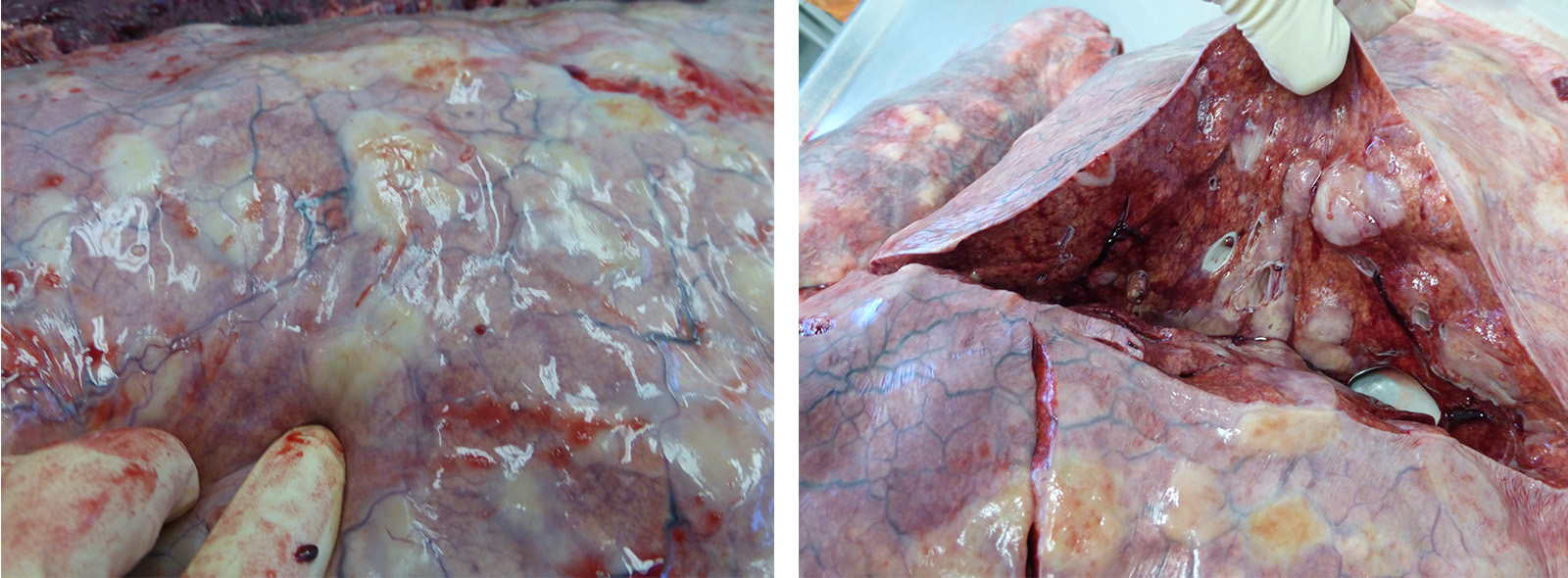
The horse is in good body condition, has good adipose reserves, and is in good postmortem condition. The oral mucous membranes are pale. There is stable foam in the trachea. There are multifocal, diffuse, round, firm nodules throughout the pulmonary parenchyma affecting approximately 50% of the lungs. The stomach and small intestine are gas filled. There is soft, wet, green ingesta in the stomach. The cecum and colon contain solid feces. The spleen is enlarged and there are multifocal, diffuse, ecchymotic, capsular hemorrhages. There is yellow concentrated urine in the urinary bladder.
Laboratory results:
Mycoplasma culture negative
Bacterial culture negative
EHV-1 PCR negative
EHV-2 PCR positive
EHV-4 PCR negative
EHV-5 PCR positive
Equine Influenza virus H3N8 PCR negative
Microscopic description:
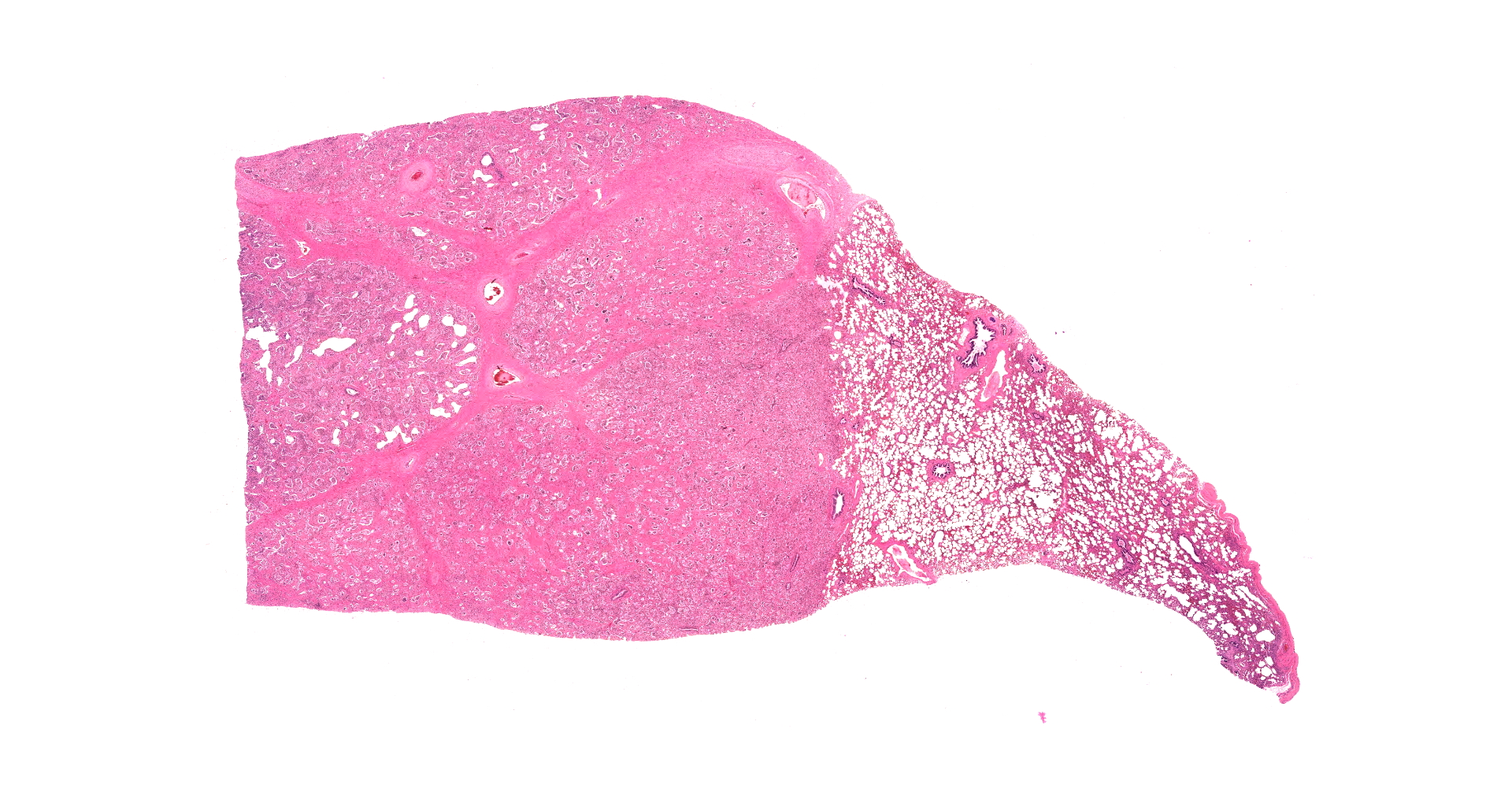
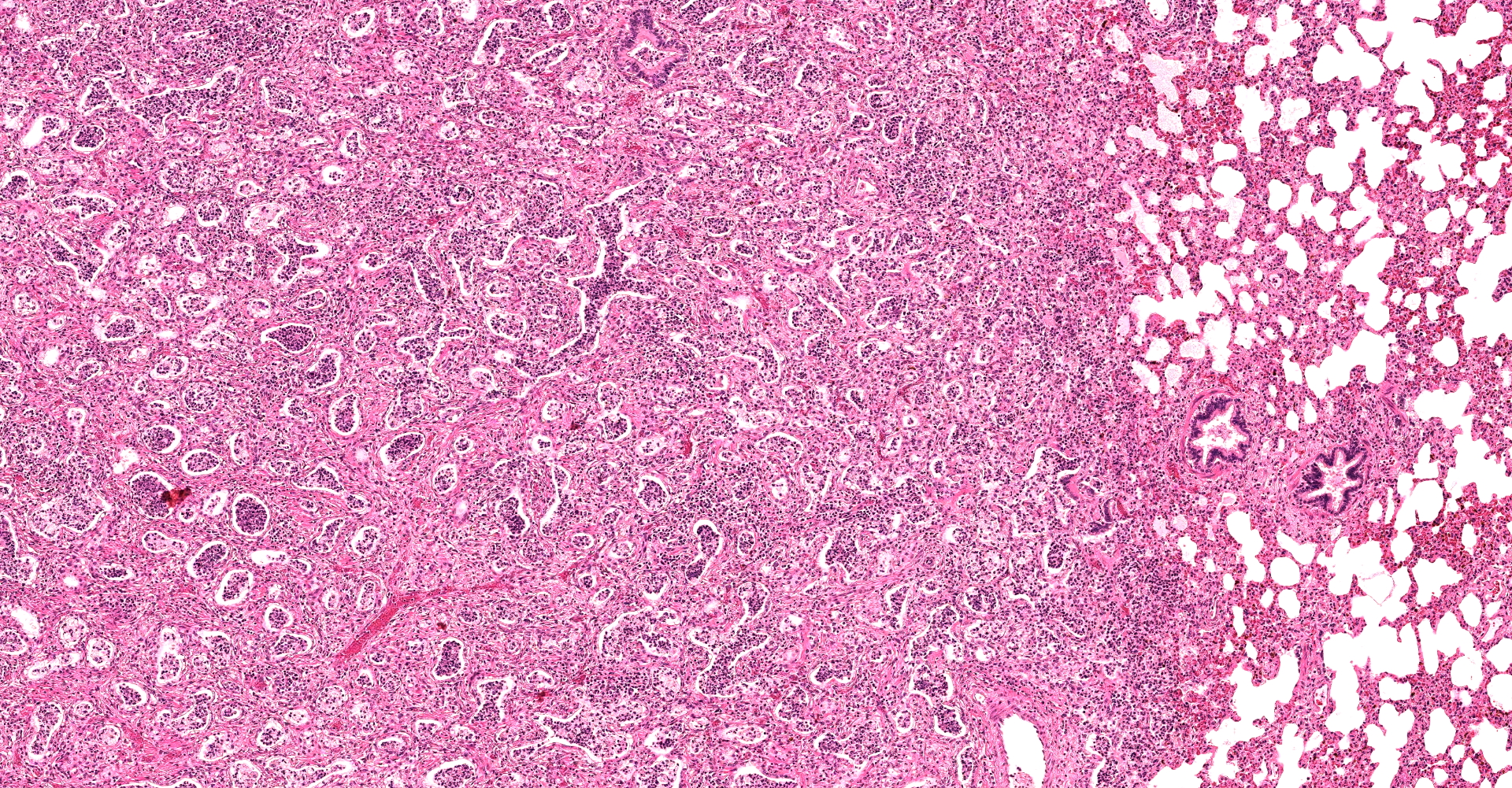
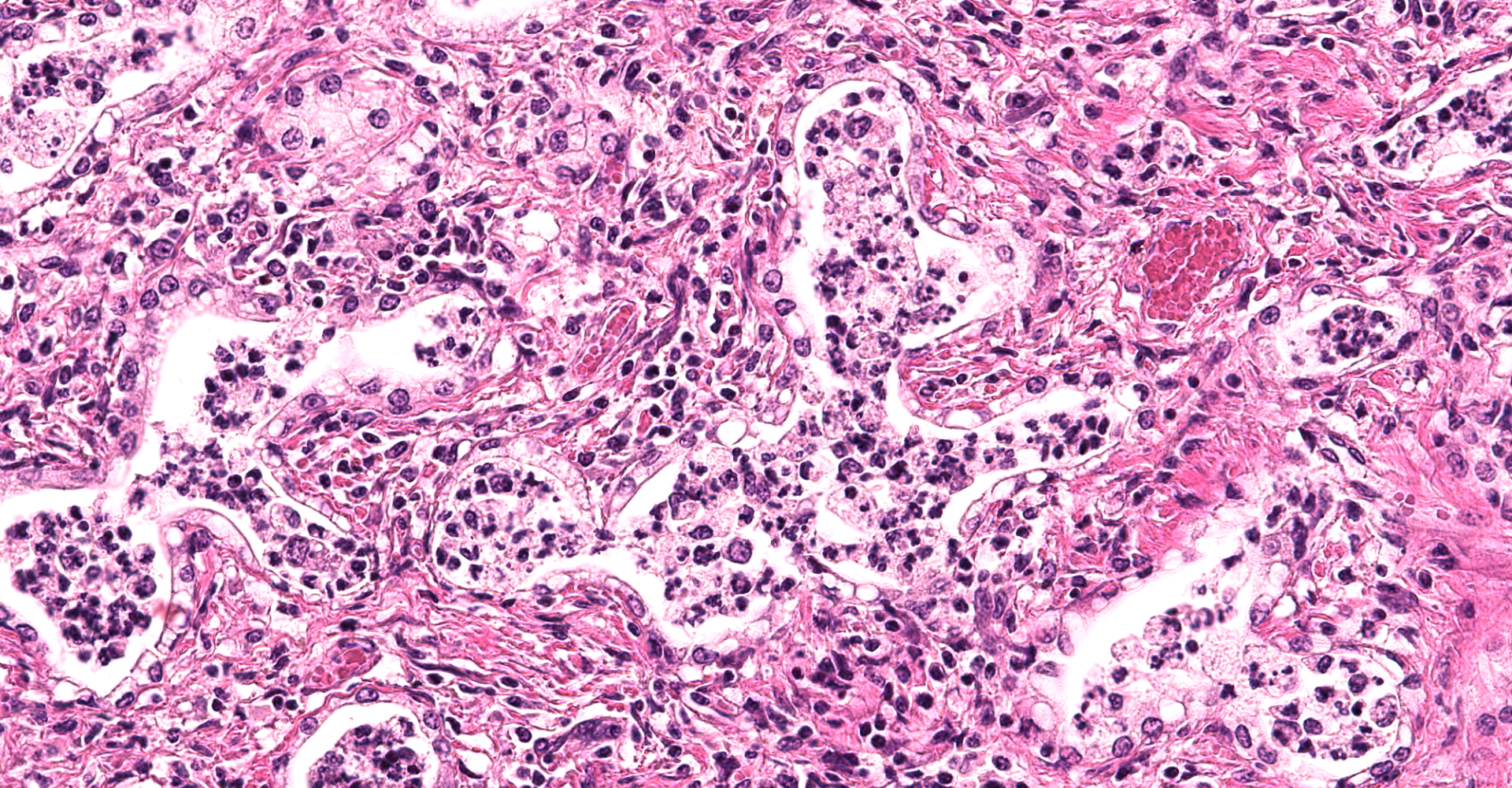
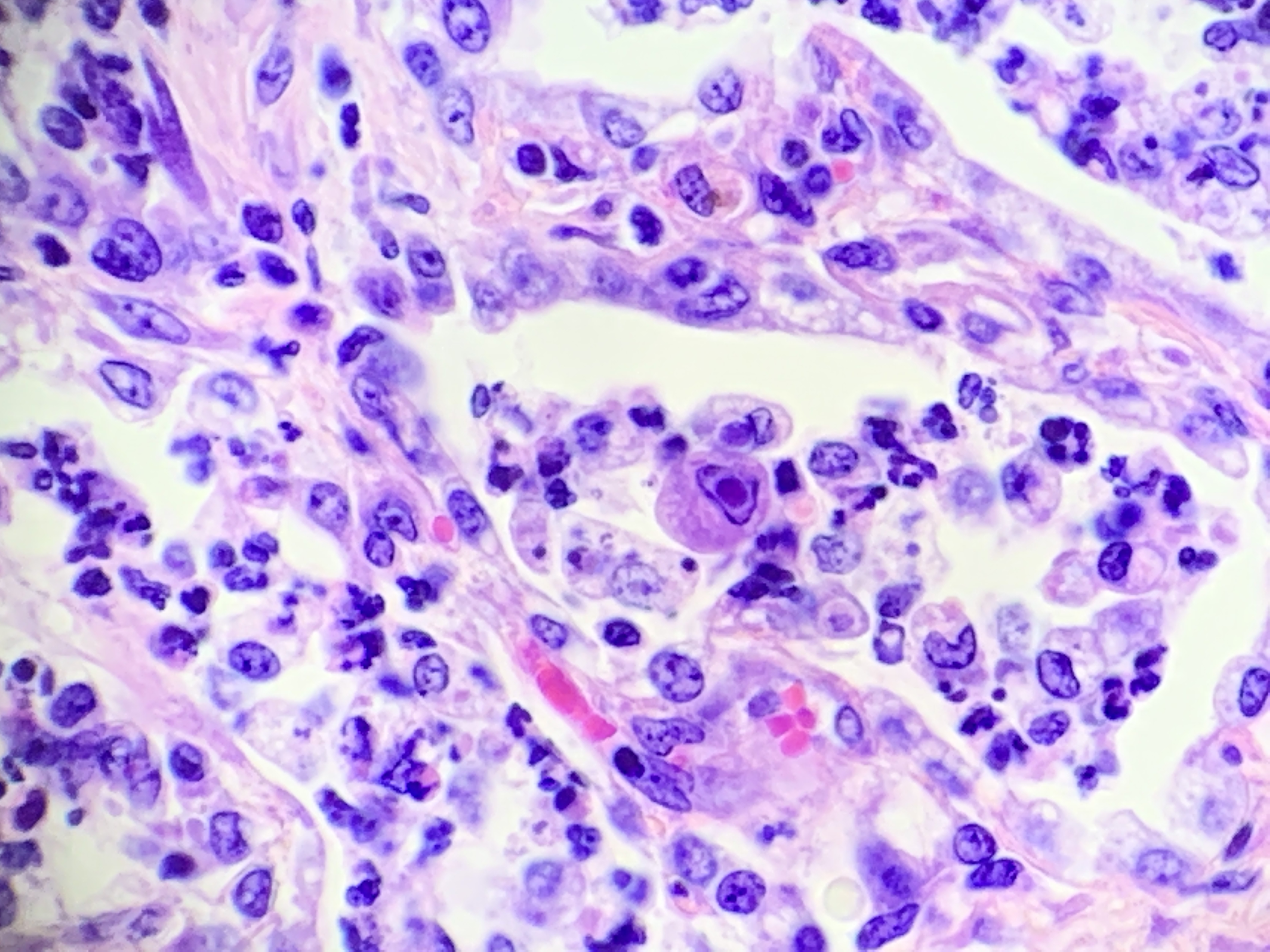
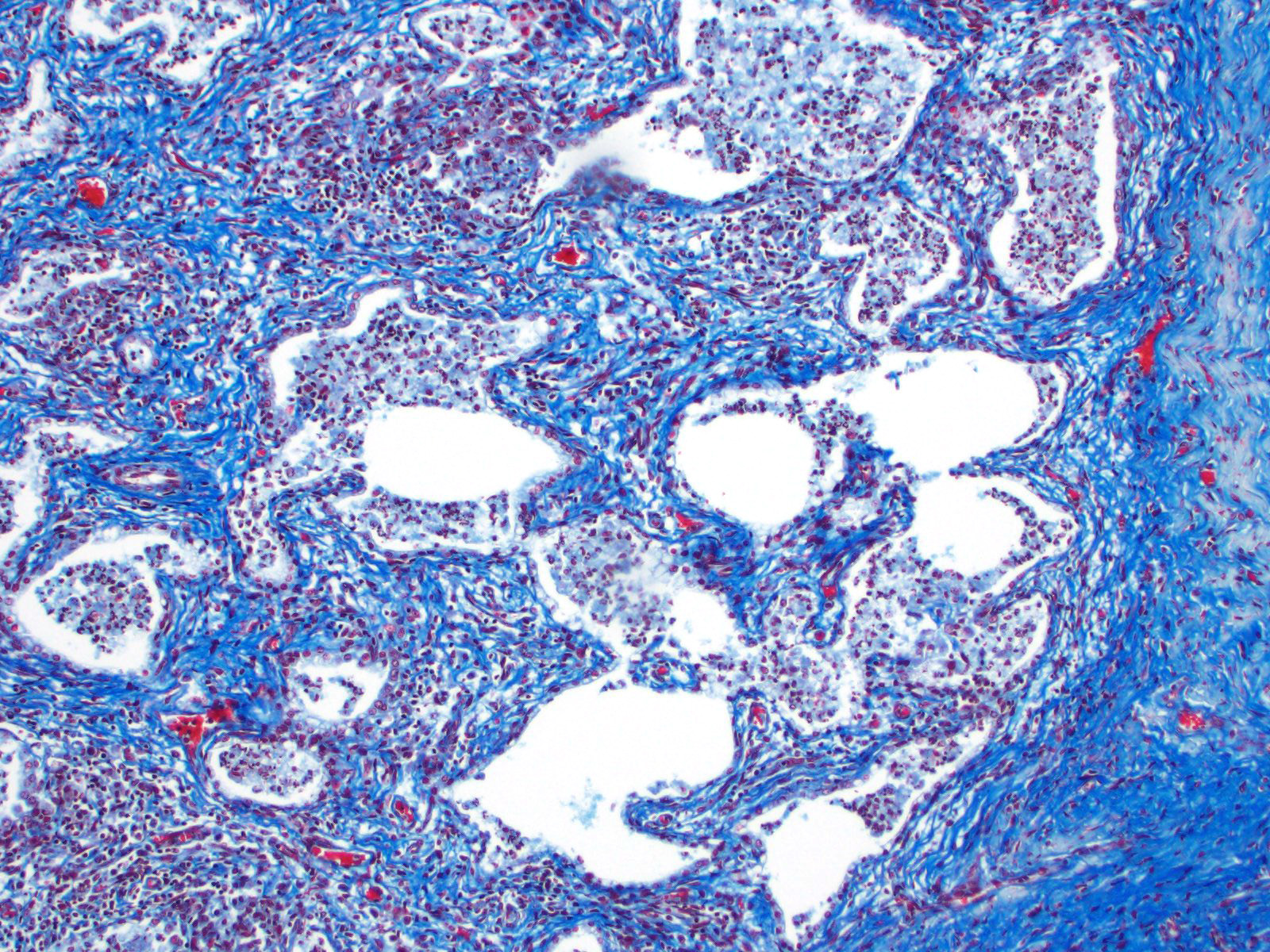
There are multifocal compressive nodules where alveolar septa are diffusely thickened by loose to mature fibrocollagenous stroma with infiltrates of scattered histiocytic cells, mononuclear cells, and neutrophils. The alveoli are often lined by cuboidal epithelium (type II pneumocyte hyperplasia) and contain variable numbers of neutrophils, histiocytic cells, sloughed epithelial cells, and/or mononuclear cells. A few alveoli contain necrotic cellular debris. Scattered intra-alveolar macrophages contain amphophilic, 4- to 6-micron, intranuclear inclusion bodies. Bronchi in the affected tissue are often lined by hyperplastic epithelium. In the less severely section of the parenchyma, there are scattered intra-alveolar hemorrhages and hemosiderin-laden macrophages. The pleura is thickened.
Contributor's morphologic diagnosis:
Multifocal interstitial pulmonary fibrosis with bronchointerstitial pneumonia and intrahistiocytic intranuclear viral inclusion bodies consistent with equine multinodular pulmonary fibrosis.
Contributor's comment:
Since 2007, the fibrotic interstitial lung disease of horses known as equine multinodular pulmonary fibrosis (EMPF) has been associated with infection by EHV-5.3,7 Equid herpesvirus 5, family Herpesviridae, subfamily Gammaherpesvirinae, genus Percavirsus, is considered ubiquitous in horse populations.3 Clinically affected horses are often concurrently infected with Equid herpesvirus 2 (EHV-2), which may suggest a synergist effect between the two viruses. The role of EHV-5 in the development of the disease is not known.3 Results of the quantitative real-time PCR reaction indicates that virus load is highest in lung tissue, transtracheal wash fluid, and bronchoalveolar lavage fluid, but virus isolation is typically unsuccessful.3 However, due to its ubiquitous nature, EHV-5 is often also found in horses unaffected by EMPF.5
In 2013, a group of researchers isolated EHV-5 from horses with EMPF, inoculated horses without EMPF, and reproduced the disease, but were unable to re-isolate the virus from the clinically affected test subjects. However, EHV-5 antigen was detected in affected lung tissue via immunohistochemistry.8 By use of PCR, EHV-5 of the inoculating sequence was detected in peripheral blood mononuclear cells (PBMCs) only twice in the experiment, whereas EHV-5 of a non-inoculating sequence was detected three times.8 There is marked genomic heterogeneity of EHV-5, which likely results in strains of variable virulence, explaining why horses can be infected with various strains over their lifetime and not necessarily develop disease.2 Genomic heterogeneity is even greater in EHV-2.2
Contributing Institution:
Kord Animal Health Diagnostic Laboratory
PO Box 40627
Nashville, TN 37204-0627
https://www.tn.gov/agriculture/consumers/pets/animal-health-diagnostic-lab0.html
JPC diagnosis:
Lung: Pneumonia, necrotizing and sclerosing, interstitial, focally extensive, severe, with marked alveolar remodeling, type II pneumocyte hyperplasia and rare intrahistiocytic intranuclear viral inclusions.
JPC comment:
Recent research on Equid herpesvirus-5 has revealed small windows into its pathogenesis in the horse. EHV5 preferentially infects alveolar cells in the lung but does not infect ciliated respiratory epithelium of the trachea or nasal airways. Additionally, similar to the human Epstein-Barr virus, another gammaherpesvirus, EHV5 induces a lytic infection of T and B lymphocytes, with peak viral antigen expression at 24- and 72-hours post-inoculation, respectively. The subsequent drops in levels of expression are hypothesized to indicate a possible latency mechanism in the host.6
While PCR and in situ hybridization has been previously used to identify EHV5 in affected horses, the first two reported cases in Japan also used an RNA-based in situ hybridization to identify viral mRNA, with positive signals identified in the cytoplasm and nuclei of alveolar macrophages in affected regions of the lung. This result suggests alveolar macrophages may play an important role in the pathogenesis of EMPF.4
The current treatment for EMPF is focused on limiting inflammation to prevent further fibrosis, specifically focusing on limiting the actions of EHV5. Corticosteroids and nonsteroidal anti-inflammatory drugs (NSAID) limit associated inflammation, and tetracycline therapy are chosen for their antifibrotic properties and their abilities to decreased metalloproteinase activity. The antiviral drug valacyclovir, which is metabolized to the active form acyclovir, is an inhibitor of viral DNA polymerase. A single case report of valacyclovir described a clinically healthy horse two years after treatment. However, in a recently conducted study in six horses with EMPF, valacyclovir did not effectively reduce the viral load as measured in peripheral blood, nasal secretions, or bronchoalveolar lavage fluid. Additional research may be warranted due to the low sample size, and that the clinical manifestation of disease is likely more complicated than strict viral kinetics.1
References:
- Easton-Jones CA, Madigan JE, Barnum S, et al. Effect of valacyclovir on EHV-5 viral kinetics in horses with equine multinodular pulmonary fibrosis. J Vet Intern Med. 2018;32(5):1763-1767.
- Forier G, van Erck E, Pronost S, et al. Equine gammaherpesviruses: pathogenesis, epidemiology, and diagnosis. Vet J. 2010;186:148?156.
- Marenzoni M, Passamonti F, Lepri E, et al. Quantification of Equid herpesvirus 5 DNA in clinical and necropsy specimens collected from a horse with equine multinodular pulmonary fibrosis. J Vet Diag Invest. 2011;23:802?806.
- Ochi A, Sekiguchi M, Tsujimura K, Kinoshita T, Ueno T, Katayama Y. Two cases of equine multinodular pulmonary fibrosis in Japan. J Comp Path. 2019;170:46-52.
- Pusterla N, Magdesian K, Mapes S, et al. Assessment of quantitative polymerase chain reaction for equine herpesvirus-5 in blood, nasal secretions, and bronchoalveolar lavage fluid for the laboratory diagnosis of equine multinodular pulmonary fibrosis. Eq Vet J. 2017;49:34?38.
- Van Cleemput J, Poelaert KCK, Laval K, Nauwynck HJ. Unravelling the first key steps in equine herpesvirus type 5 (EHV5) pathogenesis using ex vivo and in vitro equine models. Vet Res. 2019;50(13). https://doi.org/10.1186/s13567-019-0630-6
- Williams K, Maes R, Del Piero F, et al. Equine multinodular pulmonary fibrosis: a newly recognized herpesvirus-associated fibrotic lung disease. Vet Pathol. 2007;44:849?862.
- Williams K, Robinson N, Lim A, et al. Experimental induction of pulmonary fibrosis in horses with gammaherpesvirus equine herpesvirus 5. PLoS One. 2013;8(10):1?28.