Wednesday Slide Conference, Conference 18, Case 2
Signalment:
4-year-old, female, cockatiel (Nymphicus hollandicus)
History:
A 4-year-old, female cockatiel was managed by the Exotics Service over the course of one year for progressive osteolytic lesions. The patient was originally seen in the fall of 2017, for a right ulnar fracture. At that time, radiographs confirmed the ulnar fracture; in addition, mild lytic lesions were noted in multiple other bones. The animal returned in Spring of 2018. At this time, full body radiographs revealed multiple areas of bony lysis; and whole body CT confirmed wide-spread osteolysis. Her owners elected euthanasia given the poor prognosis.
Imaging:
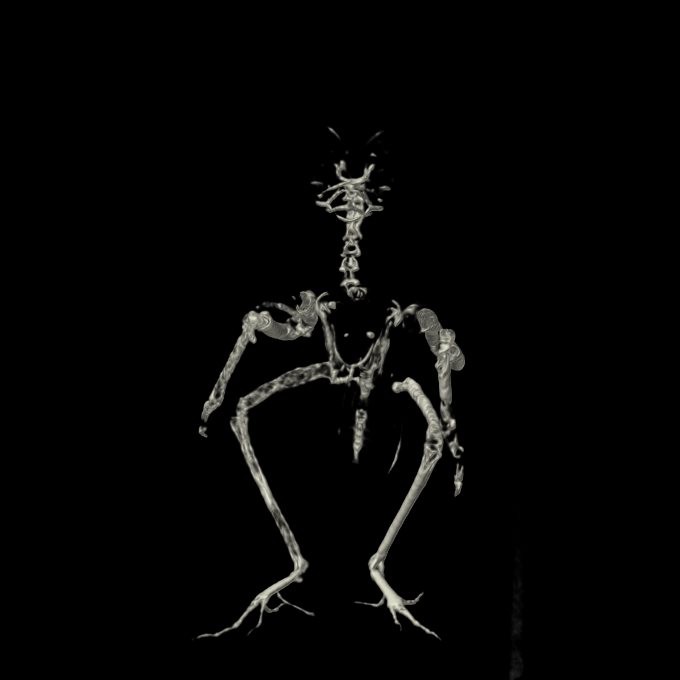
Whole body radiographs: There are multiple radiolucent expansile lesions associated with most of the long bones, with partial destruction of the adjacent cortex. These are suspected to explain the previously observed distal right ulna fracture, which currently appears mildly displaced, with new bone formation partially bridging it. A minimally displaced fracture of the proximal left ulna is also observed. Minimal soft-tissue swelling is associated with the punctiform lesions of the distal left metacarpophalangeal bones.
Computed tomographic images: The axial skeletal structures are normal. By contrast, multifocal lesions affect the appendicular skeleton (left proximal and distal ulna, left metacarpal bone III and proximal phalanx, right distal ulna, right metacarpal bone III and proximal phalanx, left distal femur, left distal tarsometatarsus, essentially the entire right femur, right proximal and distal tibiotarsus, right proximal and distal tarsometatarsus, questionably right pelvic limb phalanges): these are characterized by a thin and moth-eaten appearance of the cortices with mild irregular expansion of the bony margins. Irregularity of the bone margins is again present in the right distal ulna and left proximal ulna, and the site the previously described pathologic fractures. Medullary enostosis is not detected. Soft tissue swelling is minimal around these lesions.
Imaging Diagnosis:
1. Severe polyostotic aggressive osteolytic lesions of the appendicular skeleton with at least two pathological fractures
Gross Pathology:
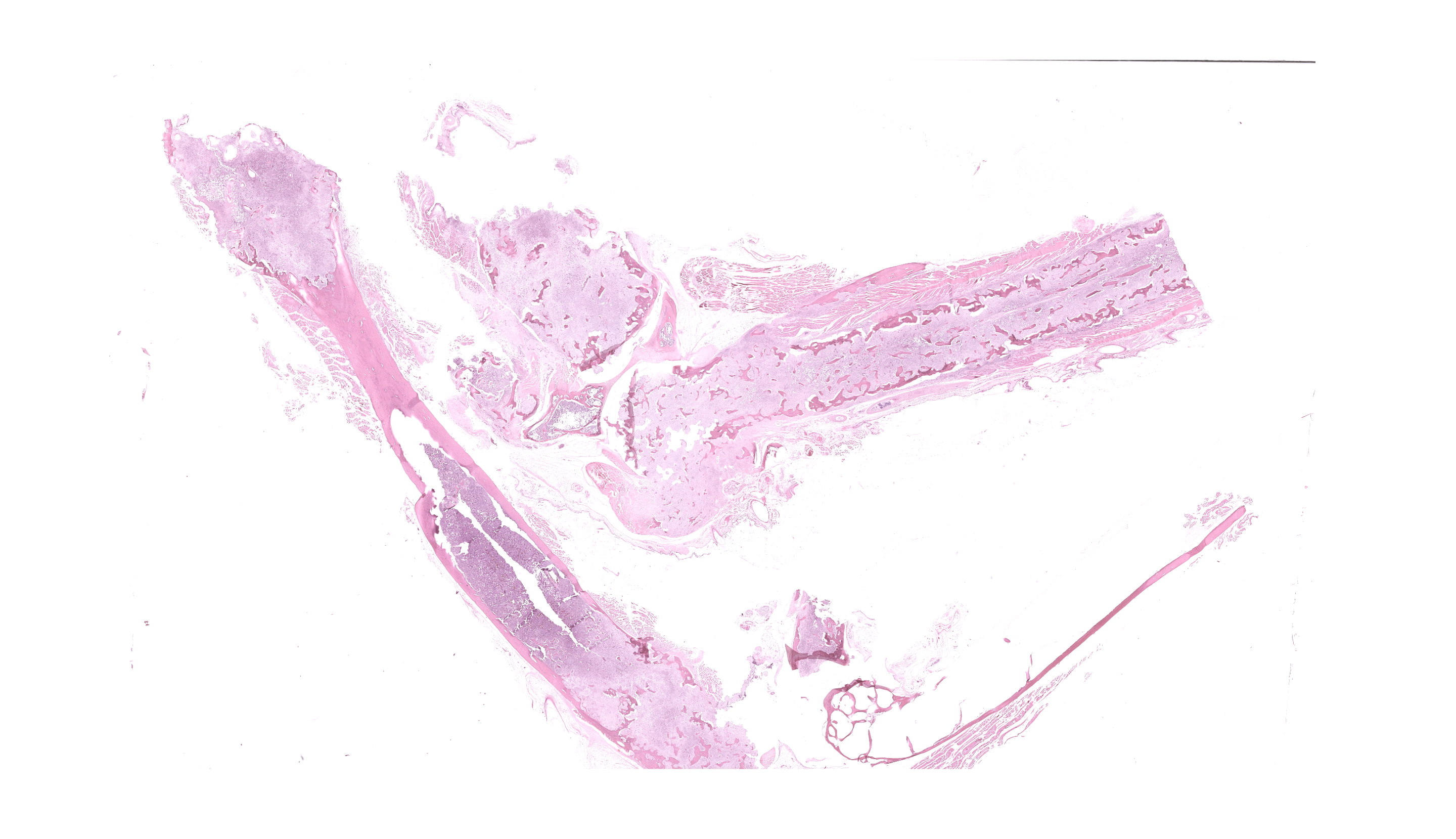
The right femur is moderately expanded with a rough cortical surface and easily fractures with minimal manipulation. The right radius has a rough cortical surface and easily fractures with minimal manipulation. The distal aspect of the right ulna is mildly expanded with a rough cortical surface. The proximal left ulna has a rough cortical surface.
After formalin fixation and decalcification, cross sections of multiple bone reveal irregularity and thinning of the bone cortices with expansion of the medullary cavity by a soft, friable, bone tissue. The surrounding skeletal muscle and soft tissues are mottled light and dark brown.
Laboratory Results:
Touch imprint cytology, Bone, right femur, ulna, and radius: Changes varied in severity between tissue imprints and one sample is comprised of blood. There is a relative mild to moderate increase in the numbers of erythroid precursors. Touch imprints contain small to moderate numbers of osteoclasts and few macrophages.
Microscopic Description:
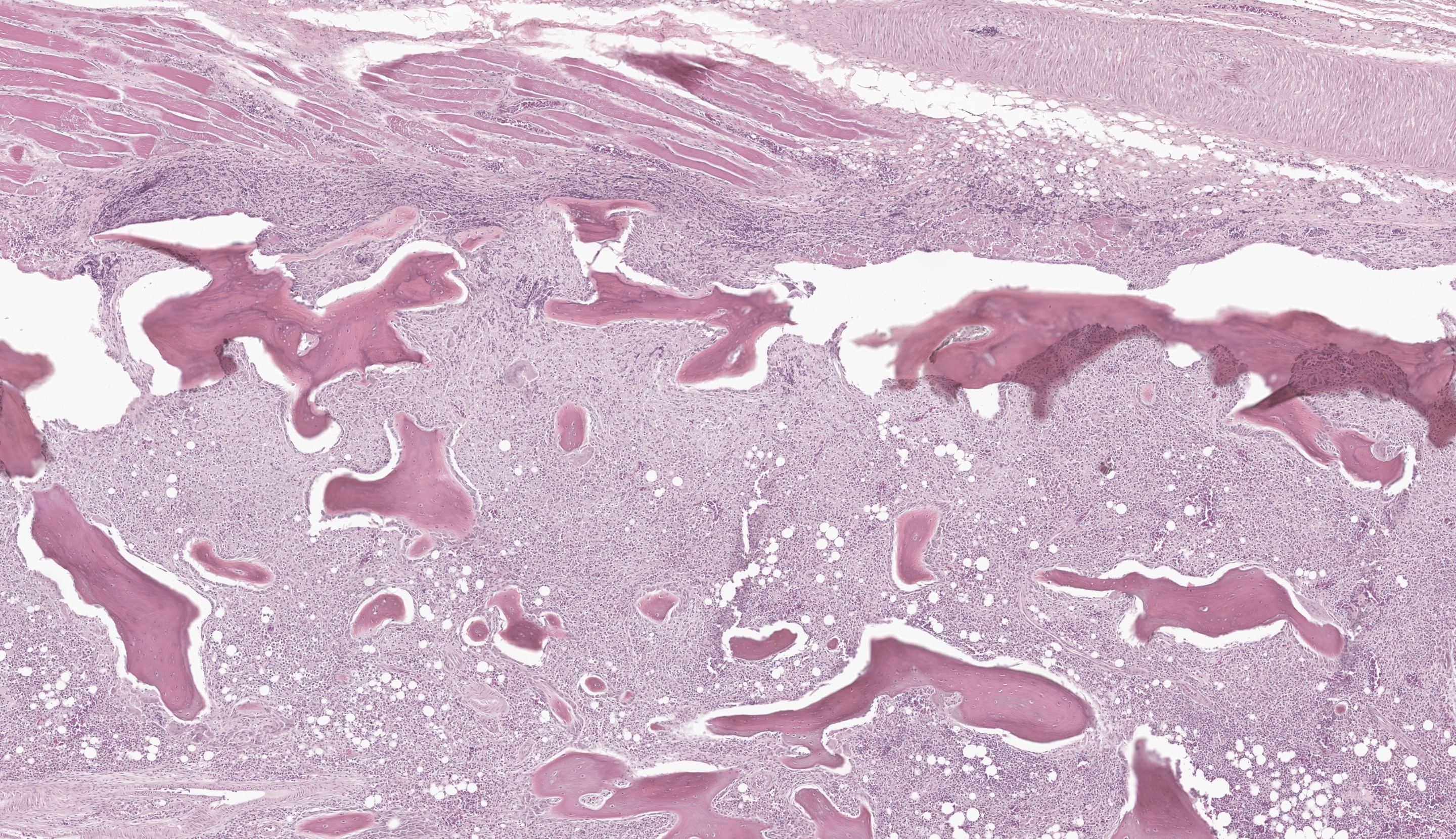
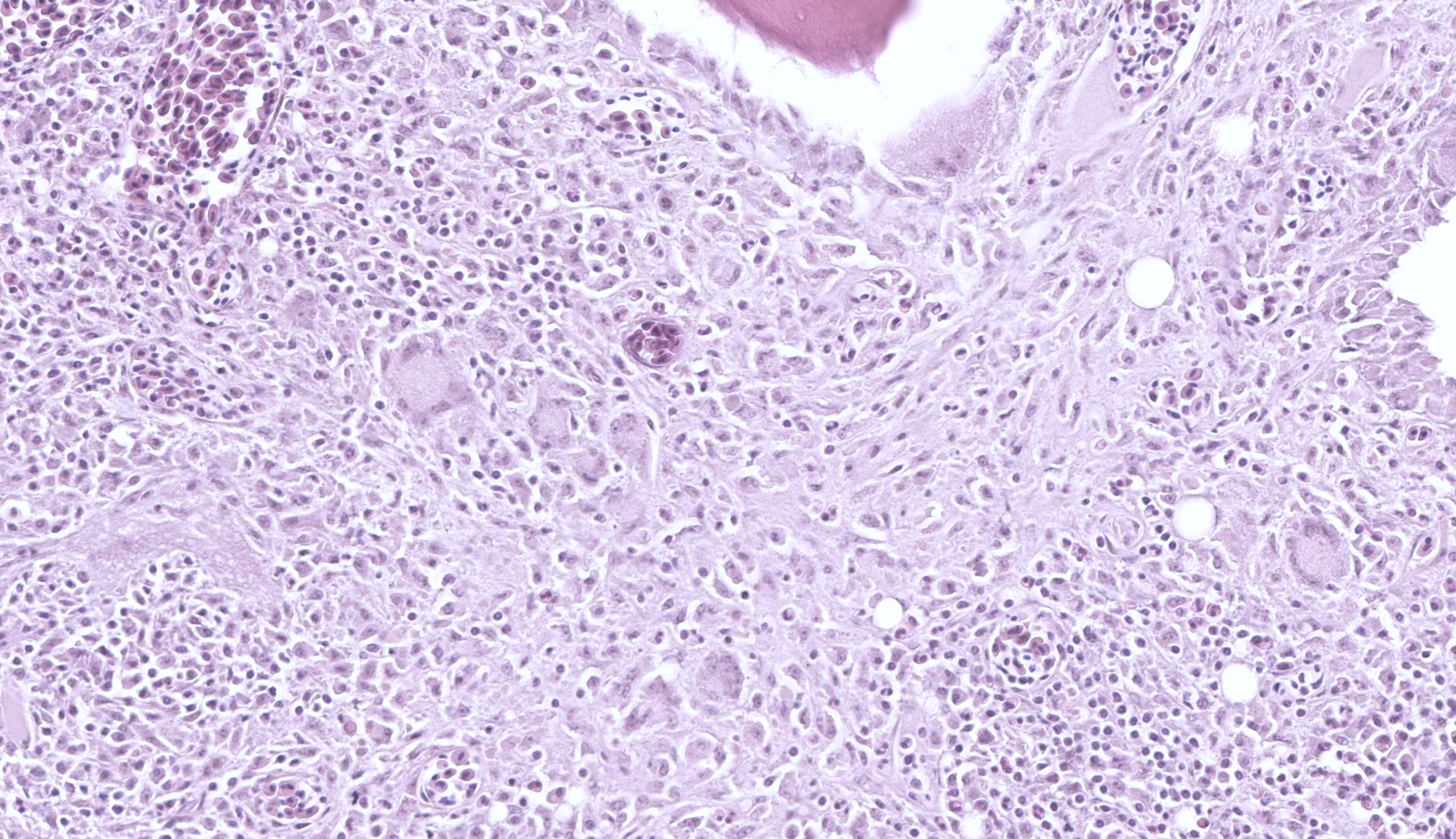
Long Bones (multiple sites): The medullary cavity is variably replaced and expanded by a dense inflammatory cell infiltrate, comprised of macrophages (including epithelioid morphology) and multinucleated giant cells mixed with heterophils, necrotic cellular debris, and lesser lymphocytes and plasma cells. In some sections, this inflammatory infiltrate entirely effaces the medullary cavity. Inflammation surrounds islands of woven bone (lined by osteoblasts) or irregular, resorbing cortical bone. The cortical bone is irregular and multifocally discontinuous where it is invaded by large numbers of inflammatory cells. The endosteal surface is scalloped and are bordered by increased numbers of osteoclasts within Howship’s lacunae. The bone margin is multifocally comprised of woven bone and there are multiple reversal lines. The outer cortical surface is irregular with prominent scalloping and the periosteum is predominantly expanded by a large numbers of similar inflammatory cells and small numbers of pleomorphic fibroblasts. Where present the subchondral bone is often irregular, thinned, and infiltrated by a similar inflammatory cell population and the joint capsule is variably infiltrated. The surrounding and intra-articular adipose tissue contains small hemorrhages and islands of a similar inflammatory infiltrate. Surrounding myofibers are variably degenerate and invaded by inflammation.
The overlying dermis contains small numbers of lymphocytes and plasma cells that commonly dissect between collagen fibrils. The epidermis is diffusely moderately expanded by compact orthokeratotic hyperkeratosis.
Fite-Faraco stains confirm small numbers of acid-fast positive, intrahistiocytic bacilli. In addition, the liver and small intestine have few, small granulomas.
Contributor’s Morphologic Diagnosis:
Cortical bone wih joints, right and left wings and legs: Osteomyelitis, periostitis, and synovitis, granulomatous, heterophilic, chronic, multifocal to locally extensive, severe with few intralesional, intrahistiocytic acid-fast positive, intrahistiocytic bacilli (consistent with Mycobacterial infection), bone resorption, multifocal, severe, remodeling, mild and periosteal fibroplasia, multifocal
Skeletal muscle, right and left wings and legs:
- Myositis, granulomatous, heterophilic, chronic, multifocal, mild to moderate
- Myofiber degeneration, chronic, multifocal moderate with mild, multifocal myofiber regeneration and multifocal hemorrhages
Contributor’s Comment:
Clinical presentation, as well as the gross and histologic findings supportive a chronic osteomyelitis with evidence of disseminated granulomatous inflammation. The presence of acid-fast positive bacilli was highly suggestive of avian mycobacteriosis, although this was not confirmed with culture or molecular testing. In birds differentials for osteomyelitis are vast and include trauma, neoplasia, lymphoproliferative disease, and infectious etiologies.10-12 Reported neoplasms in the bones of birds include osteosarcoma, giant cell tumor of bone, air sac carcinoma, fibrosarcoma, and hemangiosarcoma, as well as metastatic disease.10-12 Infectious osteomyelitis can be caused by aerobic and anaerobic bacteria (including mycobacteriosis) and fungi (e.g., aspergillosis, candidiasis cryptococcosis, and histoplasmosis).11 In this case, the presence of few acid-fast positive, intrahistiocytic bacilli was consistent with a paucibacillary mycobacterial osteomyelitis. As in this case, skeletal mycobacteriosis commonly presents as soft tissue swelling and bone irregularity.11 As the disease progresses, pathologic factures can occur.11
Mycobacterial infections are a significant cause of morbidity and mortality in numerous species, including humans.6 This diverse group of bacteria contains organisms that range from environmental saprophytes and opportunistic pathogens to obligate pathogens.14 Obligate pathogens include the tuberculosis group (M. tuberculosis and M. bovis) and the leprosy group (M. lepraemurium). Opportunistic pathogens include the saprophytes (M. fortuitum, M. smegmatis, M. chelonae, M. abscessus, and M. thermoresistibile) and the slow-growing (atypical)8 organisms (M. avium-intracellulare complex, M. kansasii, and M. ulcerans). By convention, tuberculosis refers to infections with organisms in the tuberculosis complex, while mycobacteriosis refers to those caused by atypical or opportunistic forms.6,15 Differentiation of mycobacterial species requires a combination of bacterial culture, biochemical tests, molecular techniques (PCR with subsequent DNA sequencing or Interferon-Gamma Release Assays (IGRAs)), and pigment production.6,12,13
Mycobacteria are weakly gram-positive, acid-fast positive bacilli.1-16 Mycobacteria’s lipid-rich walls make them hydrophobic, which allows them to survive in adverse environmental conditions.10,11,14,16 In other words, it is endemic worldwide, stable in the environment, and difficult to eradicate once established.1,4,11,16 Modes of transmission include the skin (typically at areas of skin barrier breakdown), inhalation, and ingestion.4, 10 In birds, oral ingestion is considered the most common.11 Infections in most species tend to be protracted and associated with a chronic wasting syndrome.11,14-16
Granulomatous inflammation is a distinct form of chronic inflammation, that is typically the result of a poorly degradable and persistent antigen, specific host responses (e.g., Th and macrophage responses), and the interplay of various pro- and anti-inflammatory mediators.15 Mycobacterium species employ multiple mechanisms to ensure survival and typically do so by entering and persisting within tissue macrophages.14,15 These include disruption of the phagosome-lysosome complex (i.e. inhibit acidification of the phagosome, phagosome-lysosome fusion), interference of cytokine synthesis and function (i.e. block injury from toxic oxygen and nitrogen intermediates), or inactivation of lysosomal enzymes.14,15 In general mycobacterial species can suppress the ability of macrophages to be activated by cytokines, especially IFN-gamma.15 Complement receptors on tissue macrophages (e.g., mannose and CD14 receptors) are the major receptors responsible for mycobacterial phagocytosis.15 Other receptors (e.g., integrin receptors, TLRs, Ig Fc receptors, CD14 receptors, scavenger receptors, etc.) are involved in the early recognition and cell signaling in response to the bacteria, which eventually leads to antimicrobial chemokine, cytokine, and metabolite synthesis.15 Mycobacteria highjack these pathways and attenuate macrophage activation in response to IFN-gamma.15 Mycobacterium avium, in particular, has a unique cell wall that prevents fusion of the phagosome to the lysosome, effectively down-regulating the killing mechanisms of macrophages.12,15 A similar mechanism is described in Mycobacterium tuberculosis.15 Migration of infected macrophages allow for discrimination of infection.14 These infections are characterized by a strong cell mediated response, where macrophage recruitment and proliferation accelerates under the influence of cytokines produced by T-lymphocytes.14,15 The chronic presentation of these cases and resulting tissue destruction are due to a combination of organism persistence and cell-mediated response and other host responses.12,15
The type of pathology and clinical disease depends on the infecting mycobacterial species, host’s immune response, host’s genetic susceptibility, dose of infection, mode of transmission, and infection stage.11 Infection is typified by granulomatous inflammation.1-16 This can manifest as diffuse visceral enlargement and/or discrete granulomas, which correlate to infiltration by a pleocellular, macrophage heavy inflammatory infiltrate.1-15 Affected macrophages can have a large amount of amphophilic cytoplasm with a fine cytoplasmic granularity but acid-fast stains are required for confirmation of intracellular bacilli.11 The number of bacteria can vary considerable and ranges from paucibacillary to abundant/florid.11,12,15 There are two main pathologic forms of mycobacterial infection: a tubercle and lepromatous form.11,15 The tubercle form is a Th1-biased immune response and is characterized by granuloma formation with a region of central necrosis surrounded by inflammation.11,15 Meanwhile, the lepromatous form is a Th-2-biased immune response and is characterized by diffuse inflammation without necrosis. 11,15
Mycobacteriosis is common in various bird species, including domestic fowl, as well as pet and exotic birds and wildlife.12 Although all species are susceptible, captive waterfowl, collection birds, tropical and ring-necked doves, Amazon parrots, brotogerids, pionus parrots, finches, and canaries are commonly reported.11 The disease can have severe economic impact and is associated with high levels of mortality and morbidity and reduction in egg production.16 In one recent studying in backyard chickens, 42% of birds died from bacterial disease.3 In this report E. coli and Mycoplasma gallisepticum or M. synoviae were the most common, but mycobacteriosis was reported in 6 cases.3 Oral route appears to be the primary means of infection in birds, with lesions typically involving the gastrointestinal tract and/or liver.11,12 Airborne and cutaneous infections can be seen to a lesser degree.12 Mycobacterium avium-intracellulare complex organism infect the intestine and due to a lack of lymph nodes, the infection subsequently disseminates easily.12 Therefore, infection is typified by a chronic, disseminated granulomatous disease in semi-mature to mature birds, although localized disease can occur (i.e. dermal or intestinal mycobacteriosis).11,12 Gallinaceous birds typically develop discrete granulomas, while psittacine and passerine species tend to develop the lepromatous form.12 The two most common mycobacterial species to affect birds are Mycobacterium avium-intracellulare complex and Mycobacterium genavense.9,10,11,12,16 Other reported species include Mycobacterium tuberculosis, M. bovis, M. gordonae, M. nonchromogenicum, M. fortuitum, M. peregrinum, M. intermedium, M. celatum, M. africanum, M. simiae, M. arupense, M. URHd0023, and M. vulneris, in addition to others.9,12 The lesions created by these species are indistinguishable from one another and co-infection can occur; thus definitive diagnosis requires culture and/or molecular testing.9,16
Avian bacterial osteomyelitis can be caused by a number of ubiquitous and opportunistic bacteria, including Mycobacterium spp., Staphylococcus aureus, E. coli, Salmonella spp., Pasteurella multocida, Streptococcus spp., Enterococcus spp., Pseudomonas spp., and Aeromonas spp.1 Mycobacteriosis in birds commonly involves the bone, and in one report 93% of avian mycobacteriosis cases had bone lesions.1, 11 Infection is clinically characterized by osteolysis (as in this case), sclerosis, or bone cysts that are most commonly located in the metaphysis of long bones, ribs, and/or sternum with or without pathologic fractures.1,12 Bacterial toxins and localized ischemia can lead to bone necrosis with sequestra formation.1 Histologic findings include severe myeloid hyperplasia and granulomatous inflammation with eventual bone marrow effacement and extension into the surrounding bone and soft tissues.11, 12 Avian granulomas associated with mycobacteriosis tend not to mineralize.1,11,12
Mycobacterium avium-intracellulare complex organisms can cause sporadic, opportunistic disease in numerous species and is typically associated with immunosuppression.14 Other important Mycobacteria of this complex include M. avium paratuberculosis (Johne’s disease), M. avium silvaticum (wood pigeon mycobacterium), M. avium hominissuis (pig and human infection), and M. intracellulare.10,14,16 Other important mycobacterial species in veterinary medicine include M. tuberculosis (human and elephants), M. bovis (domestic and wild animals, humans), M. microti (small rodents, hyraxes, llamas, pigs, and ferrets), M. africanum (rare in humans, cattle, and pigs), and M. marinum, M. chelonae, M. xenopi, and M. liflandii (reptile and amphibian species).6,7,8,15 The clustering of infections in Bassett hounds, Miniature Schnauzer, Siamese cats, Somali cats, and Abyssinian cats suggest a genetic predisposition.4,8 The cause for this predisposition is unclear but may be related to a cell-mediated immunodeficiency in either T-cells or macrophages.4 Failure to regrow hair is a unique presentation in Abyssinian cats.8 Important veterinary mycobacterial disease include Bovine cutaneous opportunistic mycobacteriosis (caused by atypical mycobacterial species), feline leprosy (M. lepraemurium, M. visibilis, and others), ulcerative dermatitis in marsupials (M. ulcerans), bovine tuberculosis (M. bovis), feline ocular mycobacteriosis (M. bovis, M. microti, M tuberculosis, M avium-intracellulare complex), and Johne’s disease (M. avium paratuberculosis).6,8,10,13,14,15 The two most common etiologic diagnoses in weedy and leafy seadragons is mycobacteriosis and scuticociliatosis, where mycobacterial infection is associated with chronic erosive and proliferative skin lesions that typically involve the snout.2 Dissemination can occur and typically involves the liver, kidney, heart, gill, and skin.2 The cause of canine leproid granuloma syndrome is unknown but mycobacterial infection is suspected given the presence of acid-fast bacilli in lesions.15 Most mycobacterial infections require treatment with long course antibiotics and/or surgical excision of affected tissues.13
Contributing Institution:
Animal Medical Center, 510 East 62nd St. New York, NY 10065. http://www.amcny.org
JPC Diagnosis:
1. Hematopoietic bones and surrounding soft tissue: Osteomyelitis, synovitis, myositis, and cellulitis, granulomatous, chronic, multifocal, severe with infractions
2. Bone marrow: Granulocytic hyperplasia, chronic, diffuse, severe
JPC Comment:
The contributor provides an outstanding summary of avian mycobacteriosis to accompany an interesting case presentation. The polyostotic nature of the lesion led some to consider neoplasia or metabolic bone disease for this case. Dr. LaDouceur noted that metabolic disease such as fibrous osteodystrophy may affect multiple bones, but more importantly is not an inflammatory lesion (inflammation was abundant in this case) and would not be expected to extend into the surrounding soft tissue. Similarly, a multifocal distribution would be unusual for a primary bony neoplasia, although neoplasia is a rule out for osteolytic lesions. Metastatic carcinomas and air sac carcinomas have also been reported in the bone of birds,17 though these are morphologically distinct from the mixed inflammatory cell population seen here.
There are a number of ancillary changes that should not be missed in this case. Although the bony changes are impressive, there are numerous small, non-displaced fractures (infractions) and degenerative changes to the joint. The adjacent joints have fibrovascular membranes that span the articular surface (pannus) that accompany villonodular proliferation of the synovium and loss of glycosaminoglycans. The hyperplastic bone marrow with marked expansion of myeloid precursors is a common concurrent change in mycobacteriosis – we estimated a myeloid:erythroid ratio of at least 4:1 where as a normal is 0.4:1 (i.e. 10x times expanded in this case). Other rule outs for extreme myeloid hyperplasia include Aspergillus sp. (see Case 4 of this conference) and avian chlamydia. Additionally, we emphasize “hematopoietic bone” in our morphologic diagnosis as there is a good example of a pneumatized (and unaffected) bone on this slide as well.
References:
- Baum RM and Hanley C. What Is Your Diagnosis. JAVMA. 2016; 248(1):51-53.
- Bonar CJ, Garner MM, Weber ES, Keller CJ, Murray M, Adams LM, Frasca S Jr. Pathologic Findings in Weedy (Phyllopteryx taeniolatus) and Leafy (Phycodurus eques) Seadragons. Veterinary Pathology. 2013; 50(3) 368-376.
- Cadmus KJ, Mete A, Harris M, et al. Causes of mortality in backyard poultry in eight states in the United States. JVDI. 2019; 31(3):318–326.
- Campora L, Corazza M, Zullino C, Ebani VV, Abramo F. Mycobacterium avium subspecies hominissuis disseminated infection in a Basset Hound dog. JVDI. 2011; 23(5):1083–1087.
- Chen H, Zhu D, Wang M, et al. Comparative Immunology, Microbiology and Infectious Diseases Amyloid A amyloidosis secondary to avian tuberculosis in naturally infected domestic pekin ducks (Anas platyrhynchosdomestica). Comparative Immunology, Microbiology and Infectious Diseases. 2019; 63:136–141
- Fowler ME and Miller RE. Zoo and Wild Animal Medicine: Current Therapy. 6th eds. Saunders Elsevier. St Louise. 2008.
- Fremond-Rahl JJ, Ek C, Williamson HR, Small PLC, Fox, JG, Muthupalani S. Mycobacterium liflandii outbreak in a Research Colony of Xenopus (Silurana) tropicalis Frogs. Veterinary Pathology. 2011. 48(4) 856-867.
- Maxie M. Jubb, Kennedy & Palmer's Pathology of Domestic Animals. 6th eds. Saunders Elsevier. 2015.
- Pfieffer W, Braun J, Burchell J, Witte CL, Rideout BA (2017) Whole-genome analysis of mycobacteria from birds at the San Diego Zoo. PLoS ONE 12(3): e0173464.
- Quinn PJ, Markey BK, Carter ME, Donnelly WJ, Leonard FC. Veterinary Microbiology and Microbial Disease. Blackwell. Ames Iowa. 2006.
- Sánchez FD, Yela IJ, Alfonseca E, Campuzano J, Morales E, Aguilar C. Respiratory tract infection caused by Mycobacterium bovis in a black swan (Cygnusatratus). Avian Pathology. 2016; 45(1):126-131.
- Schmidt RE, Reavill DR, Phalen DN. Pathology of Pet and Aviary Birds. 2nd eds. Wiley Blackwell. Ames Iowa. 2015.
- Shivaprasad HL and Palmieri C. Pathology of Mycobacteriosis in Birds. Vet Clin Exot Anim. 2012; 15:41–55.
- Stavinohova R, O’Halloran C, Newton JR, Oliver JAC, Scurrell E, Gunn-Moore DA. Feline Ocular Mycobacteriosis: Clinical Presentation, Histopathological Features, and Outcome. Veterinary Pathology. 2019; 1-12.
- Zachary JF and McGavin MD. Pathologic Basis of Veterinary Disease. 5th eds. Elsevier Mosby. St Louis. 2012.
- Zhu L, Peng Y, Ye J, Wang T, Bian Z, Qin Y, Zhang H and Ding J. Isolation, Identification, and Characterization of a New Highly Pathogenic Field Isolate of Mycobacterium avium spp. avium. Front. Vet. Sci. 2018; 4:243.
- Loukopoulos P, Okuni JB, Micco T, Garcia JP, Uzal FA, Diab SS. Air sac adenocarcinoma of the sternum in a Quaker parrot (Myiopsitta monachus). J Zoo Wildl Med. 2014 Dec;45(4):961-5.