Signalment:
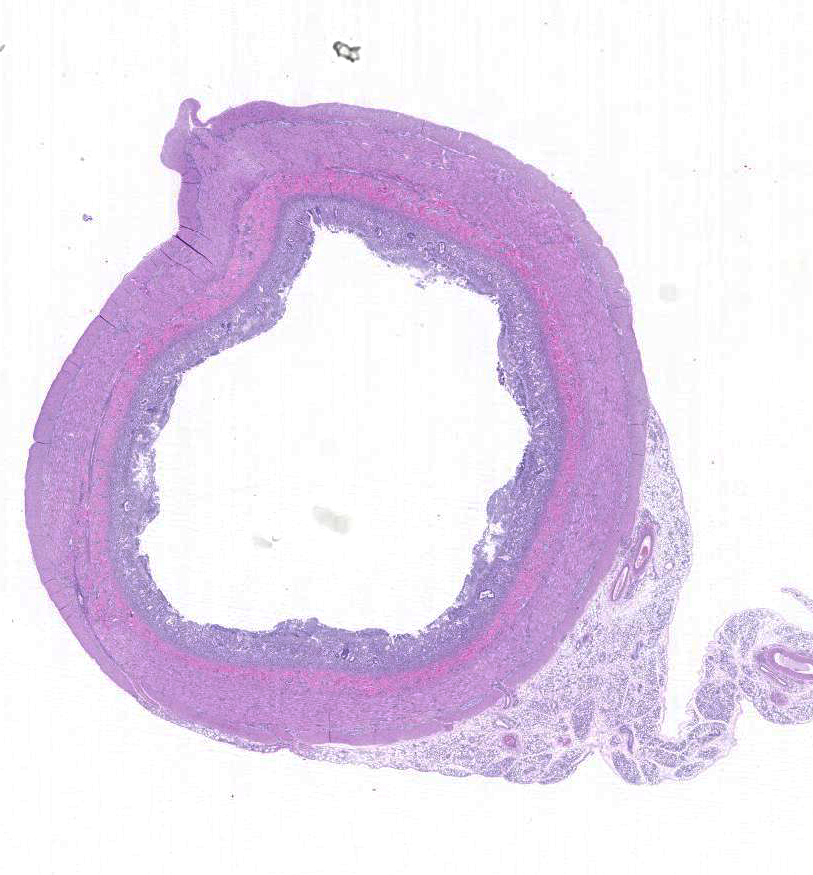
Gross Description:
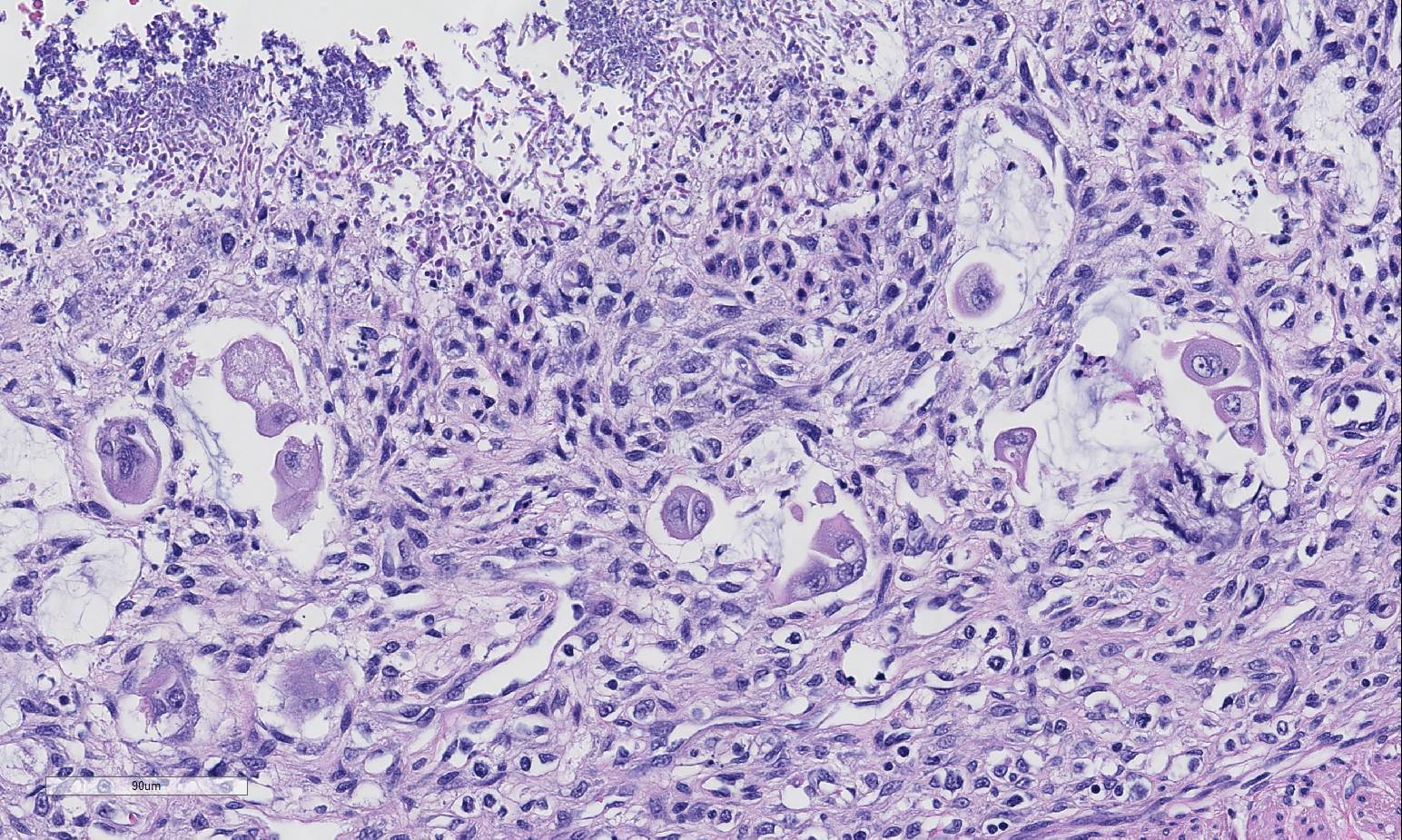
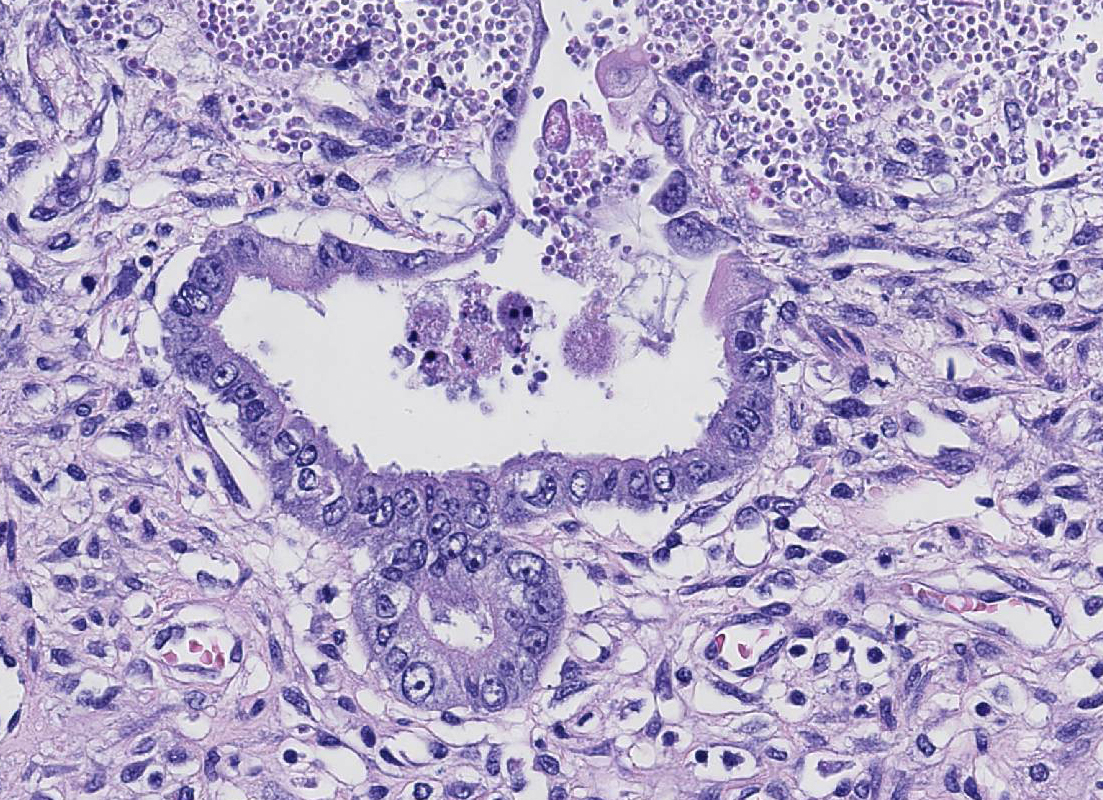
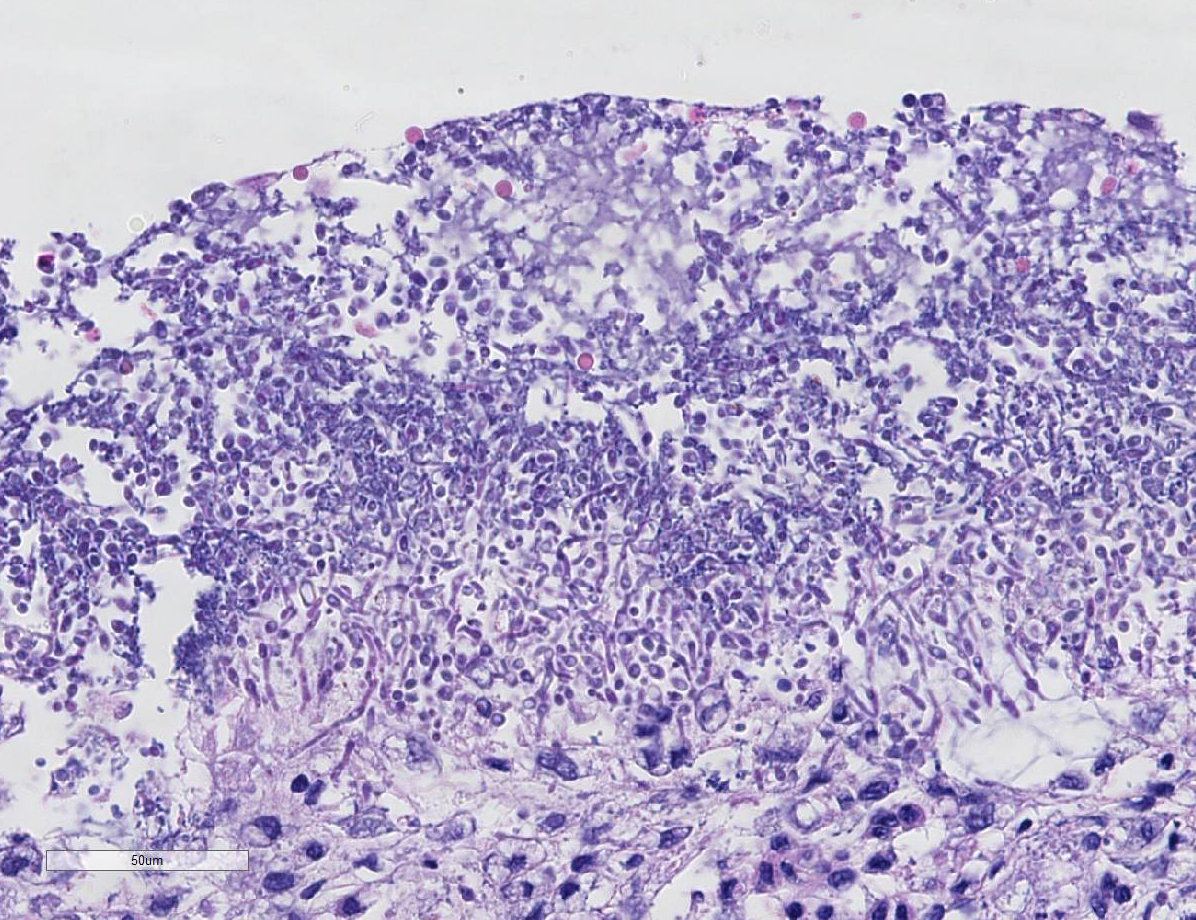
Histopathologic Description:
Morphologic Diagnosis:
1. Small intestine (duodenum, jejunum, ileum): Severe, diffuse, subacute necrotizing enteritis, with colonization of yeast (Candida presumed) and mixed bacteria
2. Small intestine (Peyers patches): Severe diffuse lymphoid depletion
Lab Results:
Condition:
Contributor Comment:
Canine parvoviruses are small non-enveloped DNA viruses that require rapidly dividing cells for replication. They are divided into CPV-1 and CPV-2. CPV-2 is one of the most common causes of infectious enteritis in dogs.5 Acute CPV2- enteritis can be observed in dogs of any breed, age, or sex. Long list of predisposed breeds is provided in referenced text.7 Since it first appeared in dogs in the 1970s, CPV-2 has a progressive series of recognized antigenic variants that are based on single amino acid substitutions in VP2 gene (most recently CPV2c have emerged and is now distributed worldwide). In dogs, etiologic diagnosis through fecal ELISA is relatively straightforward and effective in recognition of the more recent strains.8 The current vaccine series is generally effective reg-ardless of the CPV strain type. It is con-troversial whether CPV2c is more virulent in dogs, but it does decidedly have an extended host range compared to its predecessor, CPV2.
The mechanism of injury in parvoviral infection is death of rapidly dividing cells such as crypt epithelial cells, lymphocytes and bone marrow cells. Specificity for these mitotically active cells occurs because parvoviruses require a host cellderived duplex transcription template, which is only available when cells divide, during the S-phase of the cell cycle. Parvoviruses are unable to turn on DNA synthesis in host cells, so they must wait for host cells to enter the S-phase of the cell cycle before infecting these cells.4
Virus probably infects macrophages or dendritic cells migrating in the mucus layer and on the surface of mucosa.4 Virus replicates in these cells and is then spreads via leukocyte trafficking to the lamina propria of the tonsils. Here, additional mac-rophages and lymphocytes are infected and spread the virus via leukocyte trafficking in lymphatic and blood vascular systems to regional lymph nodes and systemically to the spleen, thymus, lymph nodes, bone marrow, and mucosa-associated lymphoid nodules such as Peyer's patches of the small intestine.4 Virus may also spreads as cell-free viremia in lymph via lymphatic vessels to regional lymph nodes. Experimental data suggests that virus spreads to the intestine via the vascular system and not in ingesta via peristalsis.4
Clinically, CPV in
dogs most commonly presents as hemorrhagic enteritis. However myocarditis,
thrombosis, bacteremia, and neurological disease have also been reported.4,5,7 Two cases of
cutaneous disease (erythema multiforme) induced by CPV-2 were recently
published.3,10 Typically, parvovirus infection peaks after weaning
at the age of 4 to 12 weeks, when maternally acquired antibody wanes.2 Viral tropism to
rapidly dividing cells leads to profound leukopenia and immunosuppression which
predispose infected individuals to oppor-tunistic bacterial or fungal
infections.5 Specifically, in this case, candidiasis and surface
bacterial colonization was present. Candida spp.
normally inhabit the alimentary, upper respiratory and genital mucosae of
mammals.5 Candida albicans and Candida parapsilosis
are the most common. The mechanism of injury in candidiasis is disruption and
death of cells in mucosae caused by inflammation and the concurrent
proliferation and invasion of filamentous pseudohyphae and hyphae. Candida
albicans persists in two forms: yeast (commensal) and filamentous
pseudohyphae and hyphae (pathogenic).4 Yeast persists in the
oropharyngeal cavity by adhering to and colonizing mucosae via ligand-receptor and/or
hydrophobic in-teractions. Yeast ligands include cell wall components, such as
mannose, C3d rec-eptors, and mannoproteins, whereas mucosal receptors include
fibrinogen, fibronectin, thrombin, collagen, laminin, and vitronectin-binding
proteins. The balance between commensalism and disease is tenuous and
perturbations of the mucosae and/or changes in the physiologic status of the
animal may shift this balance in favor of disease (filamentous pseudohyphal
and/or hyphal forms).4 Through
a process called morphologic (phenotypic) switching, the yeast phase switches
to the invasive fil-amentous phase. Switching appears to occur through in-ducible
chromosomal rearr-angements in the genome of the yeast in response to changes
in the mucosal environment. Switching is reversible. Under normal conditions,
the tem-perature of mucosae in the oral cavity is near room temperature (25°
C). This temperature favors the growth of yeast, whereas growth of the
filamentous phase prefers 37° C. Yeast is able to switch this temperature
dependence for growth so that the filamentous phase can grow at 25° C. 4 Pseudohyphae and
hyphae of the filamentous phase express new adhesin ligands, secrete hydrolytic
aspartyl pro-teinases that injure the mucosa, and invade the mucosae and submucosa
in which new groups of adherence receptors are en-countered. It appears that a
large group of virulence determinates are involved in the process of infection
and invasion, but no single factor accounts for virulence and not all expressed
virulence determinates may be necessary for a particular stage of infection.
4
JPC Diagnosis:
2. Small intestine, lumen: Yeasts, numerous.
Conference Comment:
CPV has also been isolated from cats as well as a few species of wild animal such as raccoons, mountain lions, and cheetahs. In general the disease caused by CPV in cats is much less severe than in dogs and has even been isolated from clinically healthy cats.6 Additionally, results from at least one study have suggested that asymptomatic cats can shed CPV for extended periods and may serve as reservoirs for infection of dogs in some cases. In that study cats were doc-umented to be shedding CPV-2a or 2b and not feline panleukopenia virus.1 CPV-2c, a more recently emerging variant, is able to infect dogs, cats, skunks, foxes and raccoons.9 It has become widespread in some areas although there is disagreement regarding the relative virulence as compared to other variants.
Conference participants noted the conspicuous absence of
inflammatory cells which is incongruent with the degree of tissue damage and
necrosis, and ch-aracteristically results from parvo-viral lymphocytolysis and
lymphoid depletion as well as lysis of myeloid precursors in the bone marrow.
Leukopenia may be extensive in severe cases but a neutrophilia with left shift
is often observed during recovery.9 Conference participants
described mucosal stromal collapse with complete, cataclysmic loss of crypts.
Canine parvovirus (CPV) is relatively unique among enteric viruses with its
predilection for intestine crypts, and this tropism may be referred to as
radiomimetic,4 referring to its imitation of the effects of
radiation. Other causes of canine enteric disease such as coronavirus or
rotavirus, both attack the villus tips and are generally associated with mild,
non-fatal disease. Canine distemper virus can infect crypt epithelium but is
not described as producing the degree of tissue destruction seen in CPV
infection. Pathogenic Clostridium spp. generally produce a more
hemorrhagic lesion with the presence of bacilli in the necrotic tissue, and the
most severe lesions will be in the large intestine.9 Conference
participants discussed the presence of yeast in this case and most agreed they
are an overgrowth or opportunistic infection, secondary to the
immunosuppressive virus and change in bacterial flora, and play a minimal role
in tissue damage. Classic gross lesions include segmental necrotizing
enteritis producing a red gut with multifocal Peyers patch necrosis. The mo-derator
commented that virus may be identified within germinal centers of Peyers
patches or mesenteric lymph nodes, but in many severe cases of enteritis virus
will be absent from necrotic intestinal epithelium at time of death due to
sloughing and loss of enterocytes. 1. Clegg SR, Coyne KP, Dawson S, Spibey N. Canine parvovirus in
asymptomatic feline carriers. Vet Microbiol. 2012; 157(1-2): 78-85. 2. Decaro N, Buonavoglia C. Canine parvovirus--a review of
epidemiological and diagnostic aspects, with emphasis on type 2c. Vet
Microbiol. 2012; 155: 1-12. 3.
Favrot C, Olivry T, Dunston SM, Degorce-Rubiales F, Guy JS. Parvovirus
infection of keratinocytes as a cause of canine erythema multiforme. Vet
Pathol. 2000; 37:647-649. 4.
Gelberg HB. Alimentary System and the Peritoneum, Omentum, Mesentery and
Peritoneal Cavity. In: Zachary JF and McGavin MD. eds. Pathologic
Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier-Saunders.
2012:322-404. 5. Greene CE. Infectious Disease of the Dog and Cat.
3rd ed. Elsevier: Philadelphia, PA; 2006. 6. Hoelzer K, Parrish CR. The emergence of parvoviruses of
carnivores. Vet Res. 2010;41(6):39. 7. Lamm CG, Rezabek GB. Parvovirus infection in domestic
companion animals. Vet Clin North Am Small Anim Pract. 2008;38: 837-850,
viii-ix. 8. Markovich JE, Stucker KM, Carr AH, Harbison CE, Scarlett JM,
Parrish CR. Effects of canine parvovirus strain variations on diagnostic test
results and clinical management of enteritis in dogs. J Am Vet Med Assoc.
2012;241: 66-72. 9. Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In:
Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals.
6th ed. Vol 2. St. Louis, MO: Elsevier; 2016:153-156, 185.
References:
10. Woldemeskel M, Liggett A, Ilha M, Saliki JT. Canine parvovirus-2b-associated erythema multiforme in a litter of English Setter dogs. J Vet Diagn Invest. 2011;23: 576-580.