Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 10
November 29th, 2017
CASE I: H12/1754 (JPC 4019375).
Signalment: 3.5-year-old, Aberdeen
angus, Bos primigenius taurus, bovine.
History: Out of a group
of 6 animals, one cow showed chronic, profuse diarrhea and severe, progressive
emaciation. The bacteriologic investigation of the feces tested positive for
acid-fast rods and negative for Salmonella. Because of the suspicion of
paratuberculosis, the cow was euthanized and submitted for post-mortem
investigation.
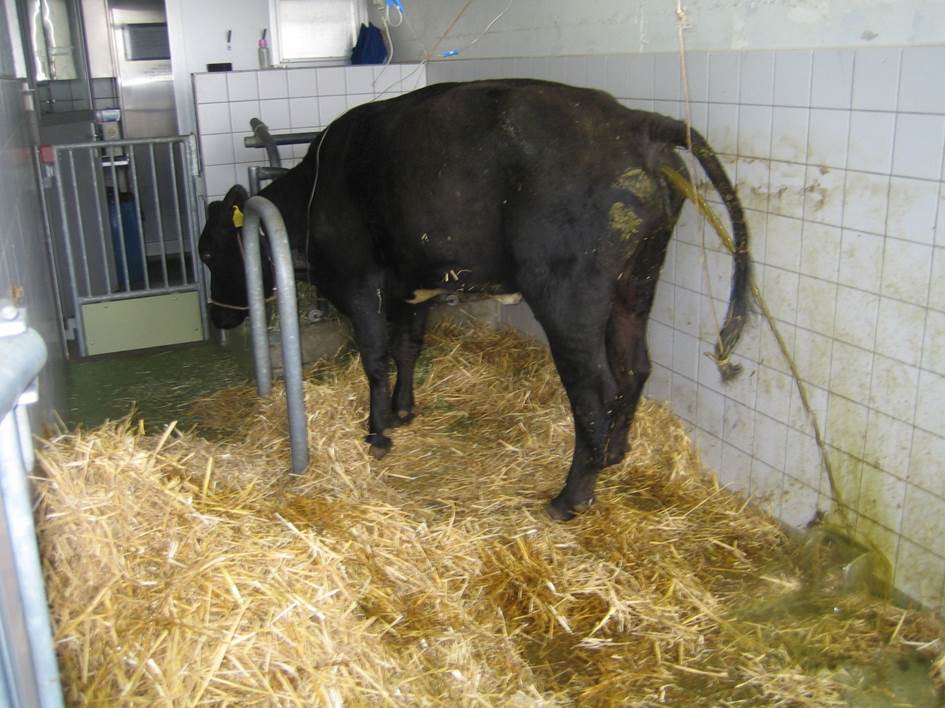
Gross Pathology: The animal was
moderately emaciated. The muscle masses were reduced, and the ribs were easily
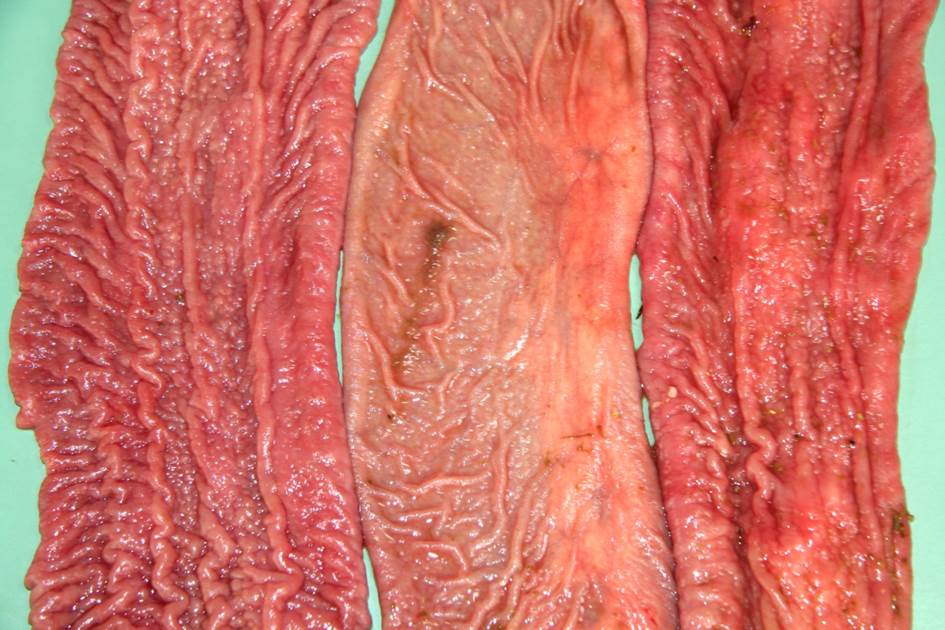
palpated. Body fat depots were present. The mucosa of the small intestine, from
the duodenum to the ileum was moderate to severe thickened, corrugated, nodular
shaped and were light brown. The large intestine content was watery and brown,
becoming slightly mucoid in the rectum. No lesions in the colonic mucosa were
noted grossly. The mesenteric lymph nodes were moderately enlarged. The rumen
pH was 6.5, and the fibers of the content were up to 15 cm long.
Laboratory
results:
Blood parameters:
Hematology:
(changed parameters)
Banded Neutrophils 0.54
109/l 0 - 0.2
Segmented Neutrophils 6.25 109/l 1.0
- 3.5
Lymphocytes 2.05 109/l
2.5
- 5.5
Monocytes 0.93 109/l 0 - 0.33
Eosinophils 0.00 109/l 0.3 - 1.5
Chemistry:
(changed parameters)
Na 116 mmol/l 135
- 165
K 1.50 mmol/l 3.0
- 6.0
Cl 68 mmol/l 90
- 110
Urea 23.99 mmol/l 1.67
- 7.50
Creatinine 183 ?mol/l 88 -
133
Bilirubin 23.0 ?mol/l 0.85 - 8.6
ASAT (SGOT) 79 IU 117
- 234
GGT 67 IU 10
- 27
GLDH 46 IU 0 -
17
Serology Bovine viral diarrhea
(during Swiss eradication program): negative.
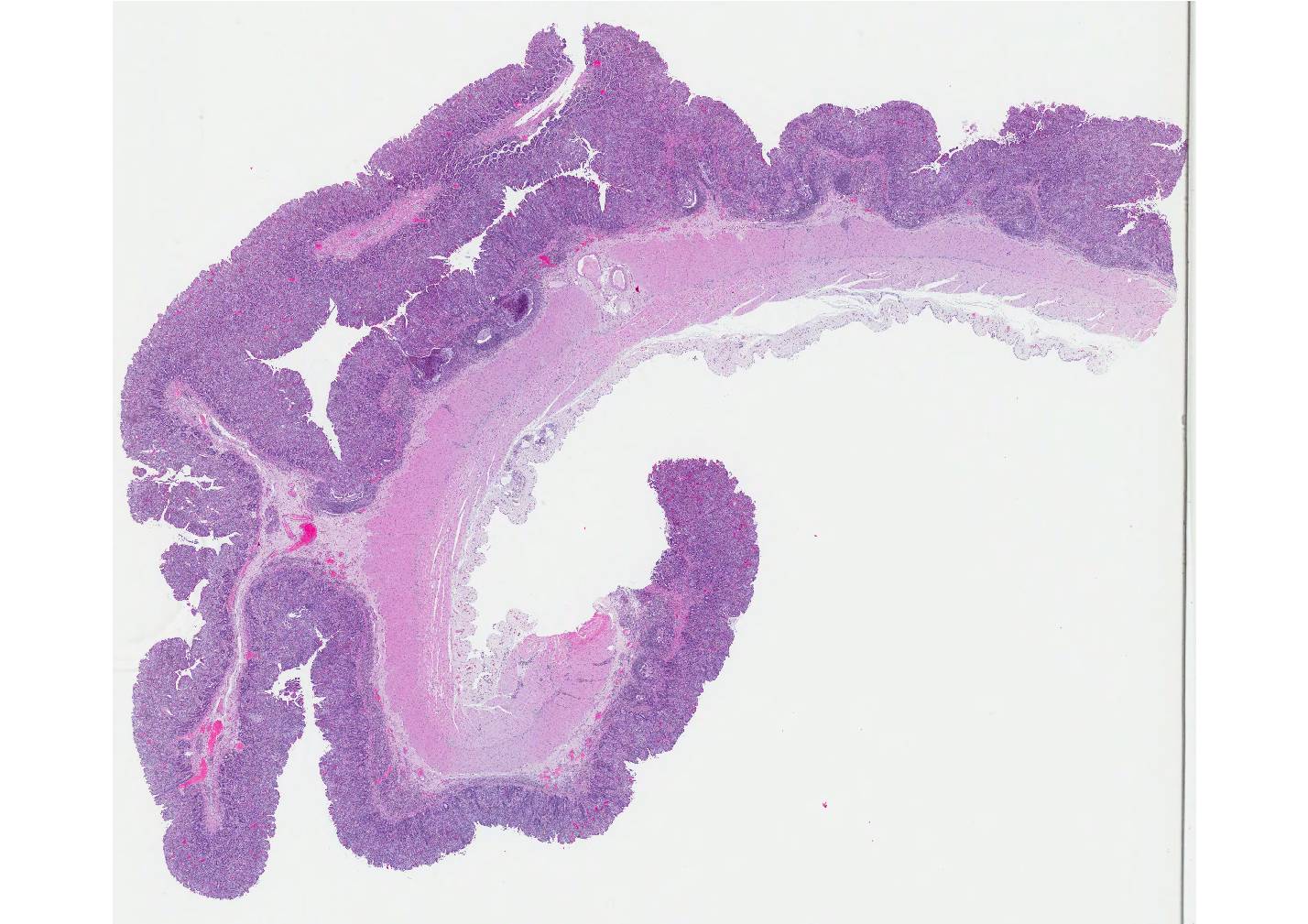
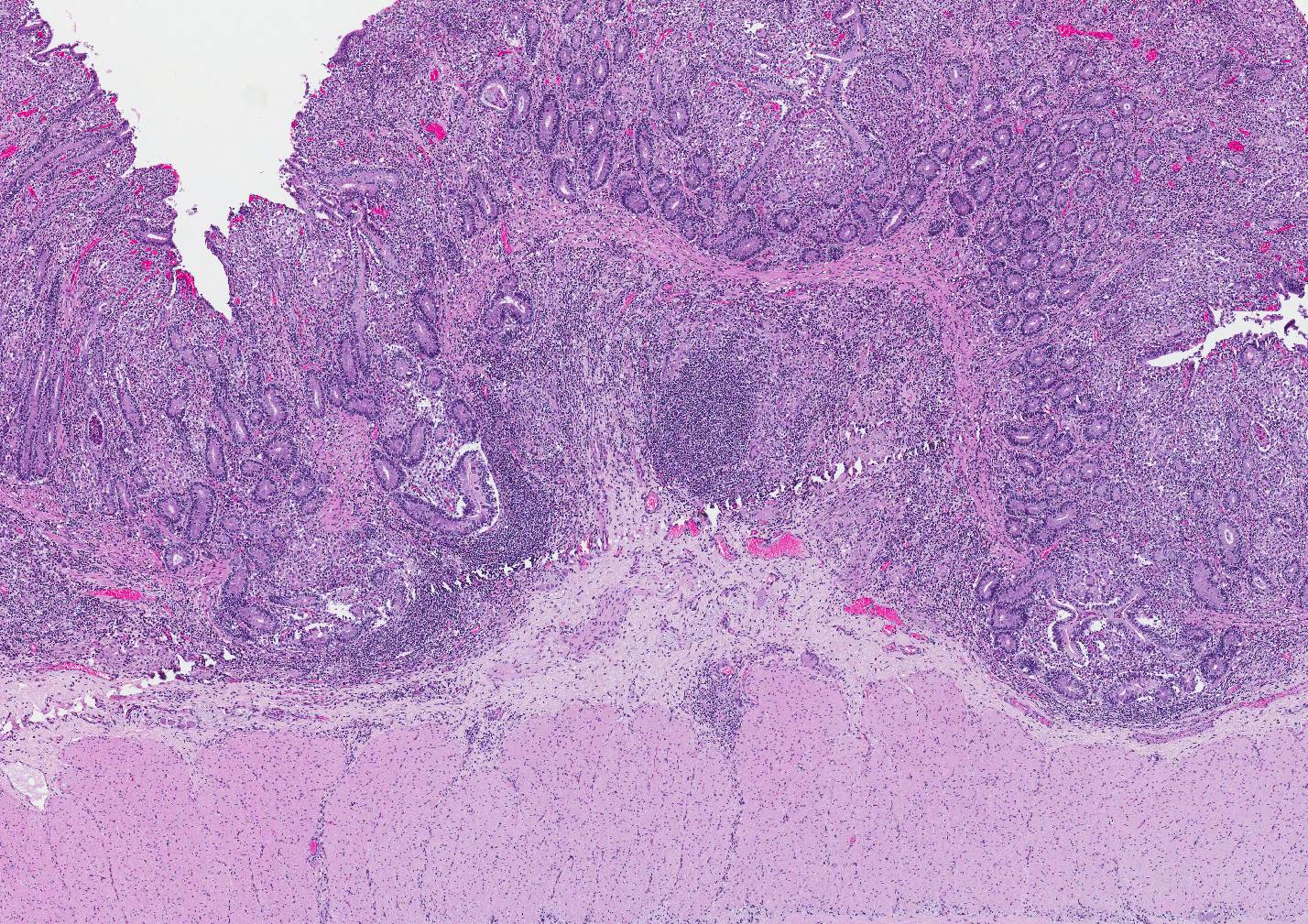
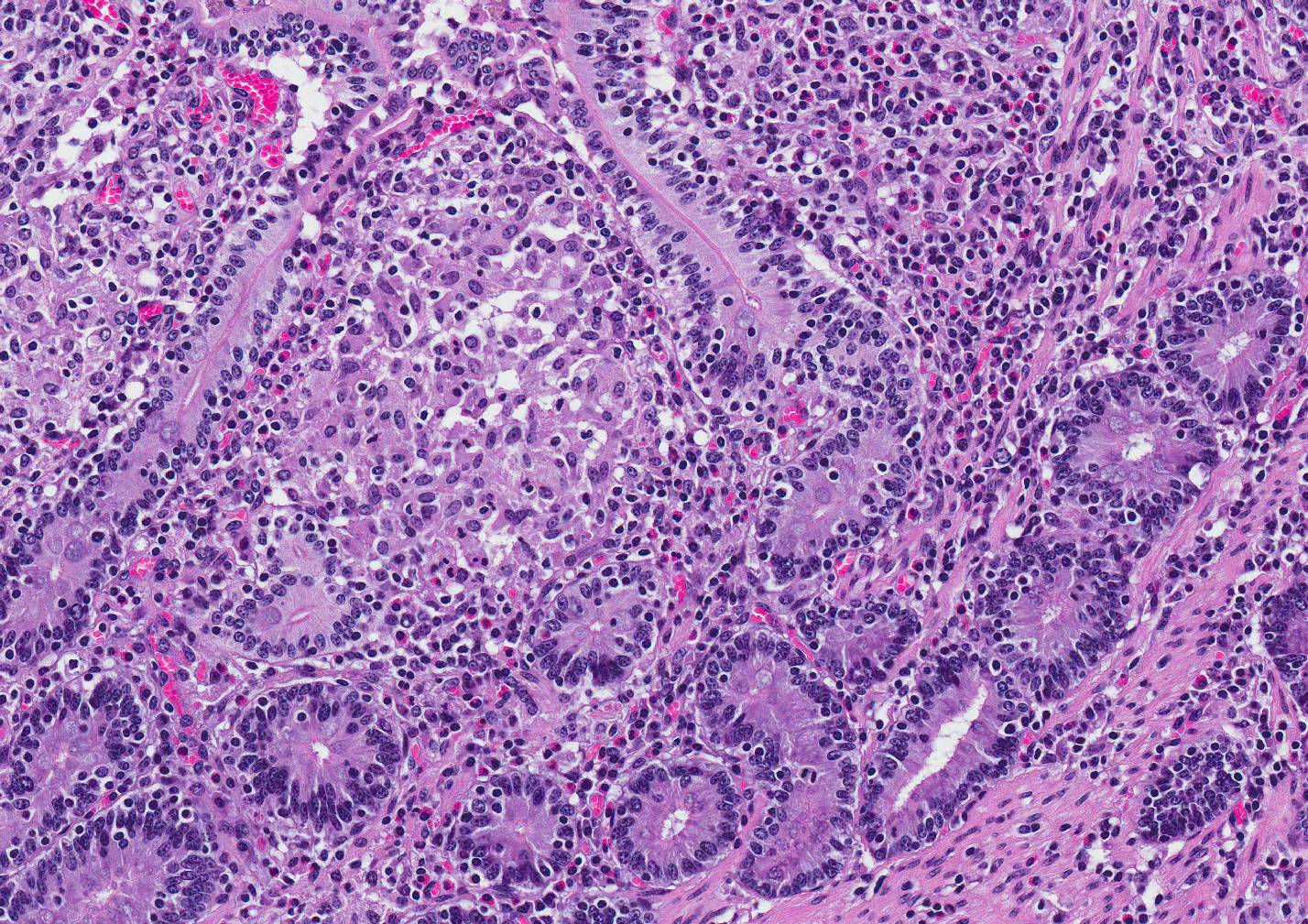
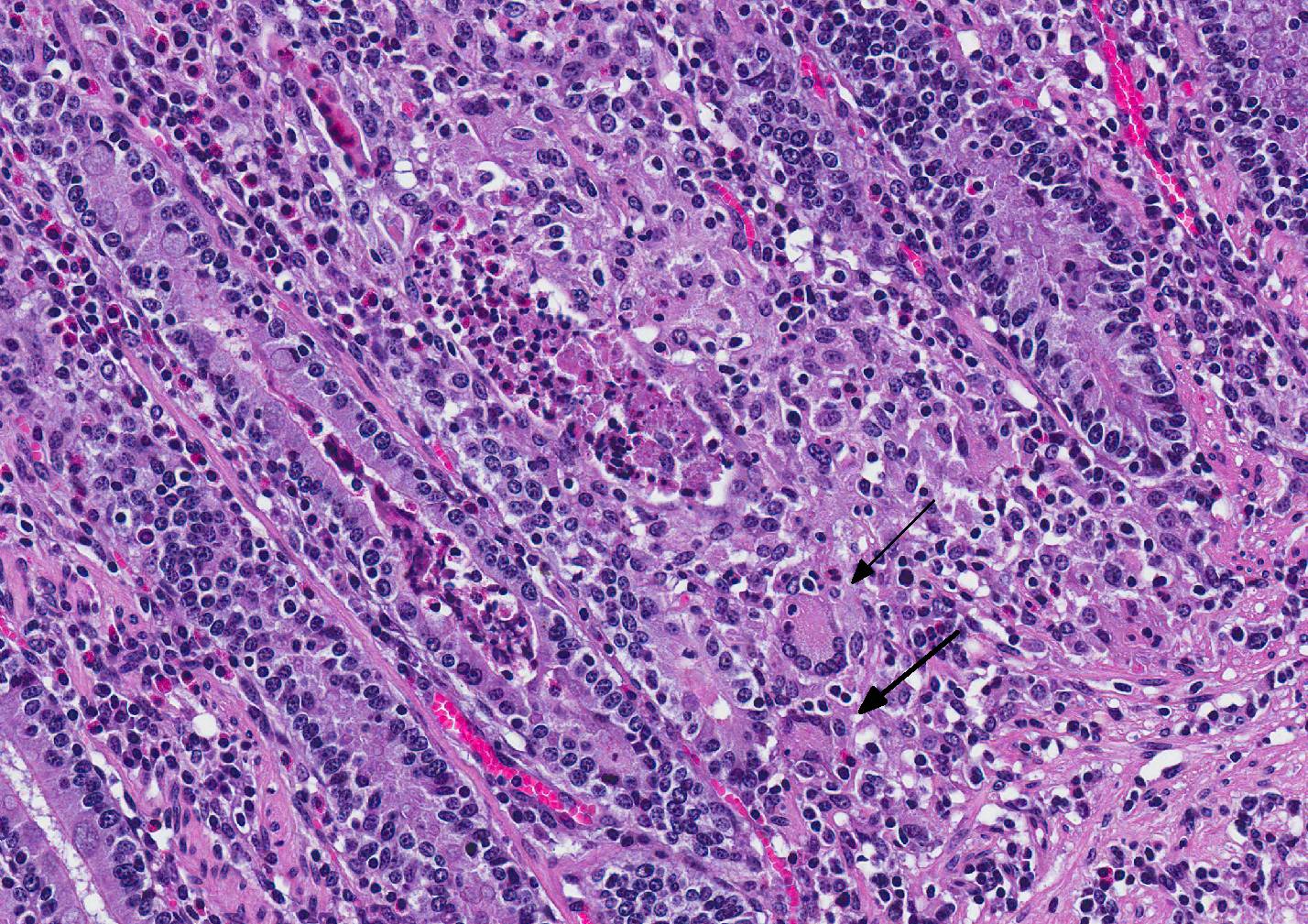
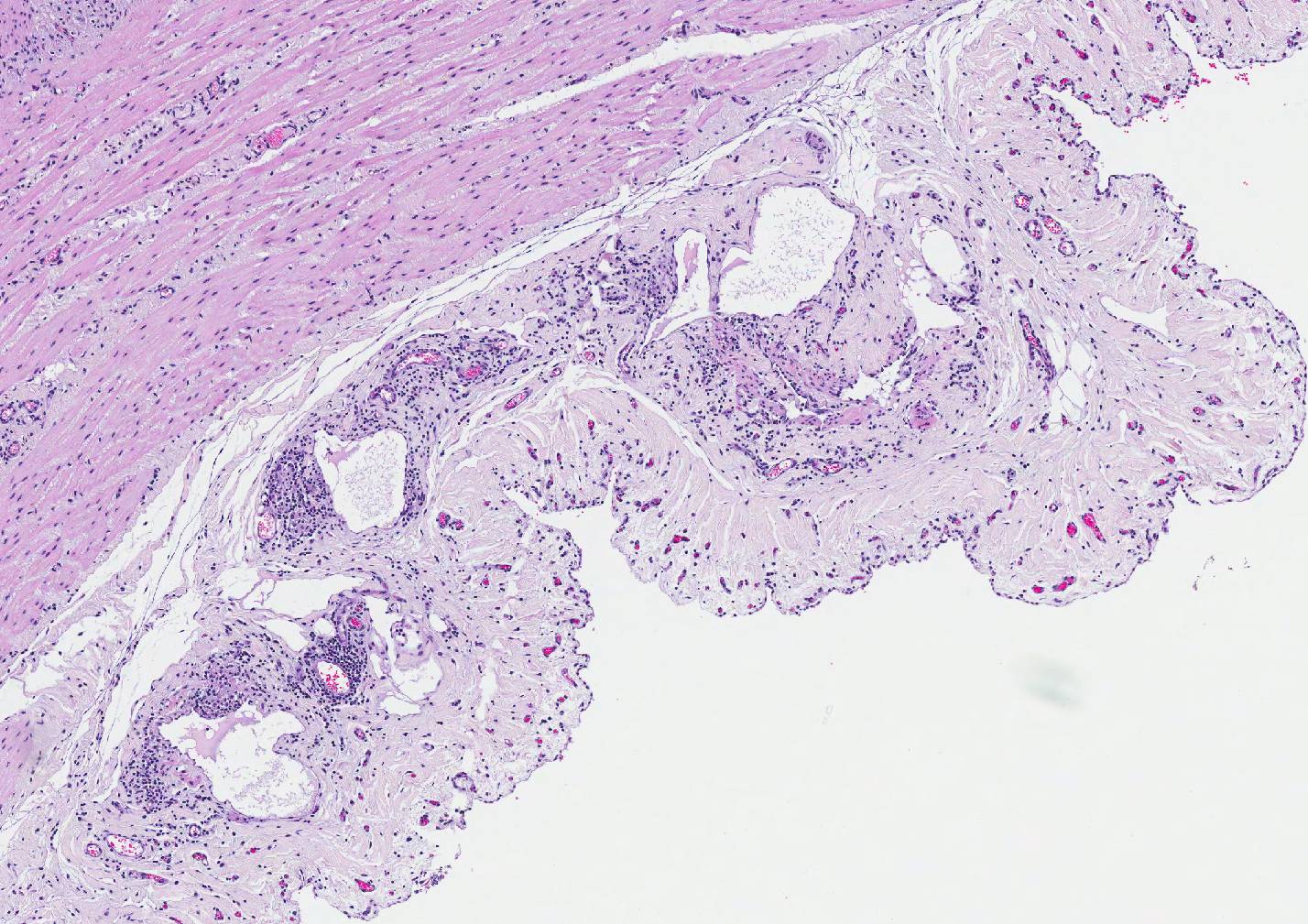
Microscopic Description: Small intestine: The intestine is thickened
and puts the mucosa in folds. About 90% of the lamina propria and the submucosa
are diffusely infiltrated by large numbers of epitheloid macrophages,
lymphocytes, plasma cells, eosinophils and fewer neutrophils with numerous
multinucleated giant cells of the Langhans and foreign body type. The
infiltration distorts and expands the lamina propria of villi. Multifocally, the crypts are moderate to severe
extended, lined by an elongated and flattened epithelium. They contain
accumulations of cellular debris, mucous and crystalline material (dystrophic
calcification) (cryptitis). Crypt epithelium piles up with cells 3-5 deep and
with a high nuclear to cytoplasmic ratio (hyperplasia). The lamina propria, the
submucosa and the serosa are diffusely widened and pale (edema). The submucosal
and serosal lymphatics are diffusely moderately to severely dilated and
surrounded by lymphocytes, plasma cells and fewer macrophages. Rarely
epitheloid macrophages and multinucleated giant cells plug the lumen of the
lymphatics (lymphangitis).
Contributors Morphologic Diagnosis:
Small intestine: Granulomatous enteritis
and lymphangitis with multinucleated giant cells, diffuse, severe, chronic.
Contributors
Comment: Lesions extended from the duodenum to the colon. During necropsy we were
impressed about the classic lesion, as it is rarely seen in our necropsy room.
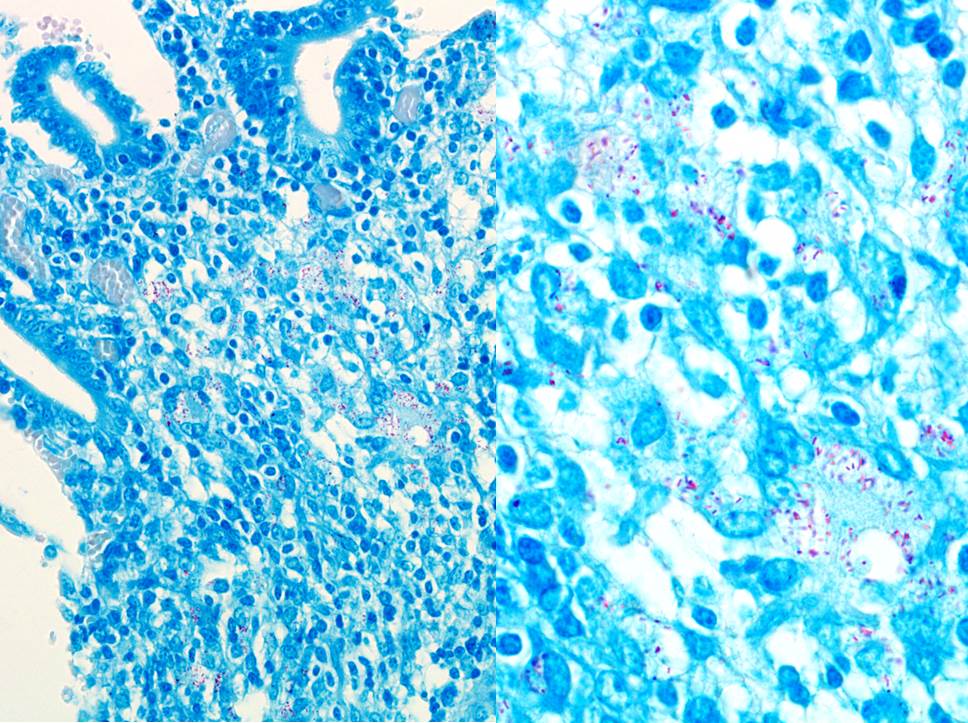
The classic histologic picture likewise was a treat. Ziehl-Neelson stained (ZN)
many acid-fast rods within the macrophages. The cow had a mild histiocytic
lymphadenitis of the mesenteric lymph nodes with ZN-positive rods as well. The
diagnosis is paratuberculosis or Johne"s disease. Although other infections can
be seen on top of mycobacteriosis (e.g. salmonellosis), further bacteriologic
investigation was not done.
Johne"s disease
(JD) or paratuberculosis, caused by Mycobacterium (M.) avium subsp. paratuberculosis
(Map), causes chronic diarrhea in ruminants. As in our case, typical
gross lesions are thickened mucosa, thrown into transverse rugae which will not
disappear when the intestinal tract is stretched. Typical microscopic lesions
were granulomatous enteritis of the small intestine and lymphadenitis of the
draining lymph nodes.
The bacteria are
taken up orally by young animals. Susceptibility to infection is greatest in
the first 30 days of life. The incubation period of JD is protracted and
clinical symptoms are usually detected in cattle 2-5 years-old. Chronic villous
involvement leads to malabsorption, protein loss and profuse chronic diarrhea
and severe emaciation. The pathogenesis of JD is best understood in cattle. It
is assumed to be similar in other ruminants, except that in sheep and goats the
enteric gross lesions are often milder. Map
can be produced in pigs. Spontaneous disease occurs in a number of free-ranging
and captive wild ruminants, camelids, rarely in equines and captive primates.
Numerous species of wild mammals and several species of wild birds are
naturally infected, though not necessarily diseased.1
Map has been suspected to play a role
in Crohn"s disease (CD), a chronic inflammatory bowel disease in humans.
Initial suggestion of Map involvement was based on the similarity of the
clinical appearance of CD and JD. Map
has been detected in multiple CD studies, but it is difficult to isolate Map from patients with CD. Genetically,
over 30 Map genes have been identified in human disease, but no specific
association has been shown so far.
JD is used as a
bovine model of CD. Studies in cattle suggest the early immune response may be
similar to the immune response to M.
tuberculosis (Mtb) during the latent stage of infection. Map affords an opportunity to examine
the immune response during the early and late stages of infection. These data
would also provide insight into how mycobacterial pathogens could contribute to
the pathogenesis of CD.2
JPC
Diagnosis: Small
intestine: Enteritis, granulomatous and lymphocytic, diffuse, marked with
villar blunting, crypt abscessation and loss, and moderate lymphangitis, Aberdeen
angus (Bos primigenius Taurus),
bovine.
Conference
Comment: Johnes
disease in cattle, sheep, and goats is caused by Mycobacterium avium ssp. paratuberculosis
(MAP) and induces granulomatous inflammation of the lepromatous (diffuse) type. The immune response is characterized by a Th2
type of adaptive immune response and appears microscopically as diffuse sheets
of macrophages and multinucleated giant cells rather than distinct granulomas
as would be expected with a Th1 response. Lesions are most common in the ileum,
colon, and mesenteric lymph nodes. Bacteria, which are often numerous, can be
identified within macrophages and extracellularly with acid-fast stains.
Johnes disease causes injury to cells in three ways: (1) lysis of epithelial
cells and extracellular matrix proteins that form cell junctional barriers in
the small intestinal mucosa, (2) dysfunction of afferent lymphatic drainage in
the small intestinal villi, and (3) lysis of monocyte-macrophage cells and
other cells within the lamina propria of infected intestinal villi from chronic
inflammatory mediators.3
Grossly, affected small intestinal walls
are thickened with a cerebriform appearance and mesenteric lymph nodes are
enlarged with coalescing areas of yellow-white caseous exudate which
occasionally mineralizes. Lymphangitis is common resulting in thickened cords
of lymphatic vessels coursing through the mesentery. Additionally, there is
marked muscle loss and wasting with intermandibular edema (attributable to
hypoproteinemia), fluid accumulation in body cavities, plaques of
mineralization and fibrosis within the tunica intima of the thoracic aorta, and
diffuse, watery diarrhea. Young animals are most susceptible, and are infected
through ingestion of the bacterium which binds to receptors on the luminal
surfaces of M (microfold) cells (which lack a mucous covering). Bacteria are then translocated across the
cell into the underlying Peyers patches and subsequently phagocytosed by
tissue macrophages. MAP requires iron for growth and secretes iron-chelating
proteins known as exochelins, iron-reductases, and siderophores as virulence
factors to acquire iron from ferritin stored in macrophages. Additionally,
mycobacterium species can: (1) inhibit acidification of the phagosome, fusion
of the phagosome and lysosome, and lysosomal enzyme activities through the
production of peroxidases; (2) block injury from reactive oxygen and nitrogen
intermediates; and (3) suppress macrophage activation by cytokines (IFN-?).3
There was apathetic debate amongst
conference attendees regarding the use of granulomatous versus
lymphoplasmacytic versus histiocytic to describe the inflammatory infiltrate in
this case, a debate which has been oft-repeated over the years, especially when
cases of Johnes disease are discussed.
In this particular case, the presence of multinucleated giant cells and epithelioid
macrophages suggest a granulomatous process, their presence however, was
restricted to the submucosa and further out, especially around lymphatics. Lymphocytes,
however, predominate in the lesion.
Ultimately, the group decided that the presence of numerous epithelioid
macrophages in the mucosa as well as the multinucleated macrophages warranted
the use of granulomatous in this particular instance.
Institute
of Animal Pathology, University of Berne
Länggassstrasse 122, Postfach 8466, CH-3001
Bern, Switzerland
http://www.itpa.vetsuisse.unibe.ch/htm
References:
1.
Brown
CC, Baker DC, Barker IK. Alimentary system. In Maxie MG, ed. Jubb, Kennedy, and Palmer"s Pathology of
Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Elsevier;
2007:222-225.
2.
Davis
WC, Madsen-Bouterse SA. Crohn"s disease and Mycobacterium avium subsp.
paratatuberculosis: The need for a study is long overdue. Vet. Immunol. Immpathol. 2012;145:1-6.
3.
Zachary
JF. Mechanisms of microbial infections. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease.
6th ed. St. Louis, MO: Elsevier; 2017:162-163.