Wednesday Slide Conference, Conference 4, Case 2
Signalment:
Adult, female, file-eared tree frog (Polypedates otilophus)
History:
A captive, adult, female file-eared tree frog (Polypedates otilophus) was found dead.
Gross Pathology:
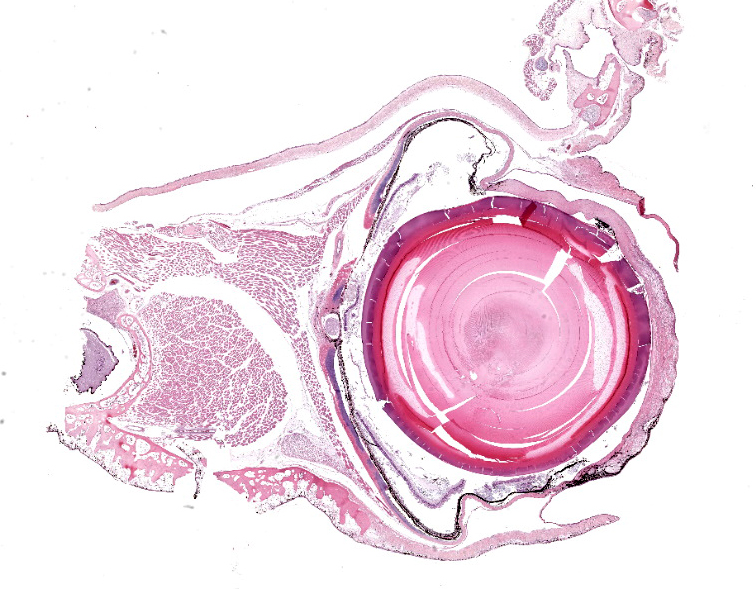
Approximately 70% of the left cornea and 80% of the right cornea contains white, multifocal to coalescing, irregularly marginated, and opaque material.
Microscopic Description:
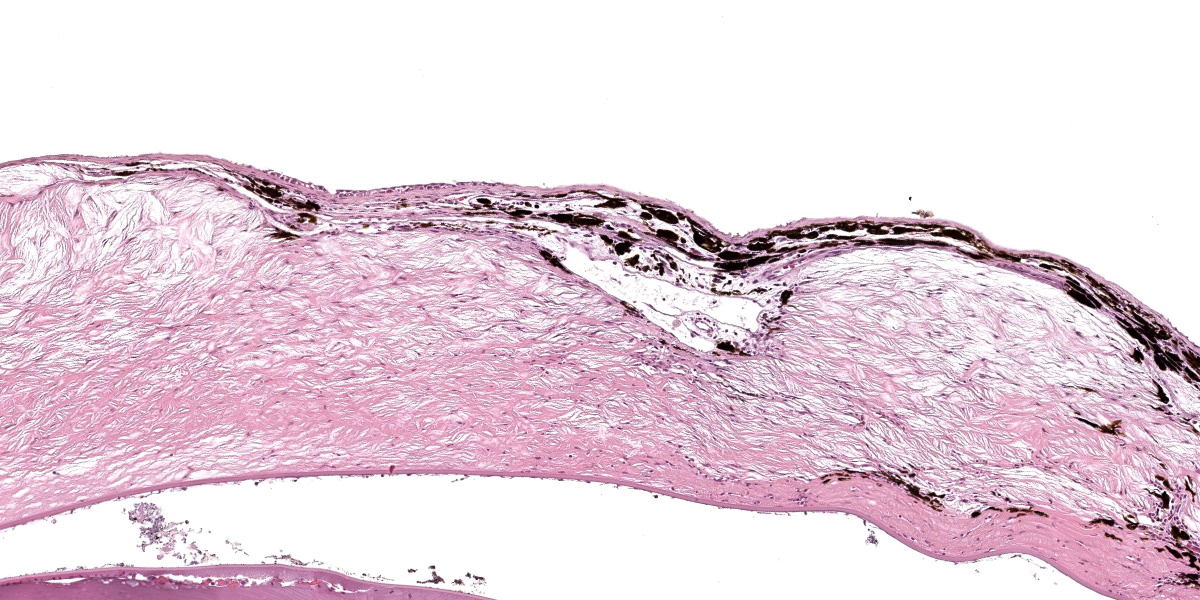
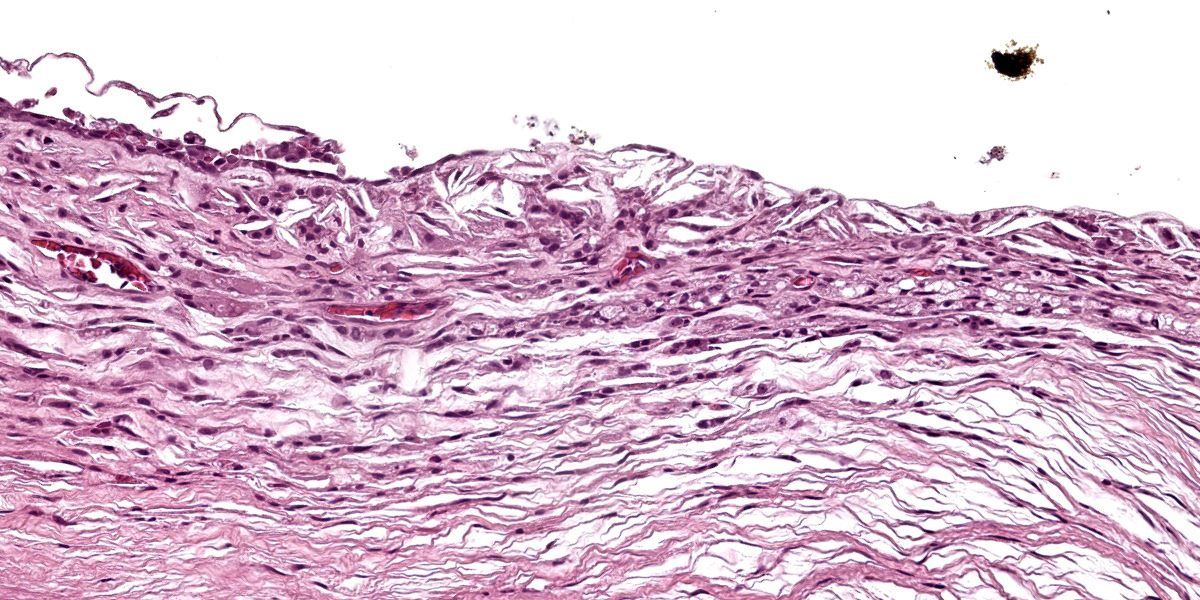
Diffusely expanding the corneal stroma, extending to the limbus, and mildly elevating the overlying corneal epithelium are hundreds of coalescing, clear, acicular clefts (cholesterol clefts), interspersed with fewer individual lymphocytes and histiocytes. Histocytes are occasionally expanded by small to moderate amounts of intracytoplasmic finely vacuolated and clear material (lipid), with an eccentrically displaced nucleus. The corneal stroma occasionally contains small-caliber blood vessels (neovascularization) interspersed with inflammation and rare individual extravasated erythrocytes. The corneal epithelium is multifocally thickened up to 5 cells thick (epithelial hyperplasia), attenuated, and rarely absent. An adjacent nerve within the section is infiltrated by small numbers of scattered lymphocytes and rare individual histiocytes.
Contributor’s Morphologic Diagnosis:
Eye, Keratitis, xanthomatous, chronic, diffuse, severe, with cholesterol clefts and neovascularization.
Contributor’s Comment:
Bilateral corneal opacities noted at gross necropsy in this captive, adult, female file-eared tree frog (Polypedates otilophus) correlated to microscopic evidence of severe corneal lipid deposition. Given the species affected and the apparent absence of underlying ocular pathology, a diagnosis of lipid keratopathy was favored.
Corneal lipid deposition, also referred to as lipid keratopathy or corneal lipidosis, is a commonly encountered ocular disorder of captive amphibians.2 While initially reported in Cuban tree frogs, lipid keratopathy has since been identified in multiple anuran species.1,2,5,6 The disease presents clinically as a circumferential accumulation of white infiltrative material extending across the cornea over the course of weeks to months.6 Lesions begin as small white foci at the corneal limbus and as disease advances can extend centrally to involve up to one-half of the corneal circumference.5,6 Vision may become compromised in severe disease, affecting thermoregulation and ability to detect food.8 In this case, the frog remained in good body condition, suggesting it was still able to find food despite the presence of corneal lesions.
Corneal lipid deposition is typically bilateral, and the corneal surface may become raised, thickened, and irregular from lipid deposition, grossly appearing as white plaques or nodules.3,8,9 Early lesions are characterized histologically as small numbers of cholesterol clefts and foamy macrophages within the corneal stroma. As disease progresses, lipid accumulation can become associated with variable degrees of inflammation, neovascularization, and fibrosis.4 Underlying ocular disease is typically not identified within affected cases.6 Special stains including oil red O and Sudan black B can be used to highlight lipid within the corneal stroma.6
The inciting cause for corneal lipid deposition in captive anurans is suspected to be nutritional and associated with variations in diet lipid composition. In one experimental study, corneal lipid deposition was found to be more prevalent in frogs fed high-cholesterol diets.6 Furthermore, captive frogs fed both normal and high-cholesterol diets were found to have a higher serum total cholesterol than wild frogs, suggesting there may be an association with diets in captivity and disease development. Some reports also identify an increased prevalence of corneal lipid deposition in female anurans.2,5
In some species of amphibians, corneal lipid deposition has been reported in conjunction with disseminated xanthomatosis.1 Xanthomas are non-neoplastic masses comprised of cholesterol and associated granulomatous inflammation, which form via extravasation of lipids.10 Xanthomas have been reported in a wide range of taxa, can occur in multiple tissues, and can be associated with elevations in serum cholesterol.5 Anurans diagnosed with xanthomatosis have been reported to have associated lesions involving the cornea, central nervous system, peripheral nerves, multiple visceral organs, and periarticular and digital soft tissues.2,4 In this case, a xanthoma was identified in the choroid plexus.
Contributing Institution:
Wildlife Conservation Society, Zoological Health Program
https://oneworldonehealth.wcs.org
www.wcs.org
JPC Diagnosis:
Eye, cornea: Lipidosis, chronic, focally extensive, severe, with multifocal ulceration, vascularization, and pigmentation.
JPC Comment:
This second case is descriptively simple which admittedly is a rarity for many of the eyeballs we cover in conference. The numerous clear acicular clefts within the cornea is an obvious feature of this case which nicely correlate to the submitted gross image.. The migration of pigmented epithelial cells from the limbus is both a response to ulceration and an adaptive response to chronic irritation. Conference participants looked carefully through this section, but found little else of note. Changes in other parts of the globe were largely ascribed to autolysis, although a focal area of hyperplasia of the retinal pigmented epithelium suggests an area of antemortem retinal detachment. Although hemorrhage was not a feature corneal neovascularization in this case, it has been documented in this entity in other frog species.3
Although lipid keratopathy is an important differential in this species, there are other differentials for corneal opacity (or ‘white eye’ in general) across species that merit brief discussion.7 Corneal dystrophy may be acquired or inherited and reflects abnormal lipid metabolism of corneal endothelial cells, stromal, cells, or epithelial cells (keratocytes). Affected animals may not have an underlying serum lipid abnormality; this has been described in dogs (among others, beagles, Siberian huskies, and collies) and rabbits (New Zealand White). Familial hyperlipidemia as a cause of lipid deposition is well known in Schnauzers and Watanabe rabbits. Other potential rule outs include hypopyon, and rarely, anterior staphyloma. Finally, corneal granulation tissue and/or mineralization secondary to inflammation and hypercalcemia should also be considered.
References:
- Carpenter JL, Bachrach A Jr, Albert DM, Vainisi SJ, Goldstein MA. Xanthomatous keratitis, disseminated xanthomatosis, and atherosclerosis in Cuban tree frogs. Vet Pathol. 1986; 23(3):337-339.
- Holmberg BJ. Ophthalmology of exotic pets. In: Maggs DJ, Miller PE, Ofri R, eds. Slatter’s Fundamentals of Veterinary Ophthalmology. 4th ed. St. Louis, MO, USA: Elsevier; 2008: 427-441.
- Moore BA, Gjeltema J. Once in a blue moon: Lipid keratopathy and intrastromal hemorrhage in a Mission golden-eyed tree frog (Trachycephalus resinifictrix). Vet Ophthalmol. 2019 Nov;22(6):933-936.
- Pessier AP. Amphibia. In: Terio KA, McAloose D, St. Leger J, eds. Pathology of Wildlife and Zoo Animals. 1st ed. London, UK: Elsevier; 2018:921-951.
- Russell WC, Edwards DL, Stair EL, Hubner DC. Corneal lipidosis, disseminated xanthomatosis, and hypercholesterolemia in Cuban tree frogs (Osteopilus septentrionalis). J Zoo Wildl Med. 1990; 21(1):99-104.
- Shilton CM, Smith DA, Crawshaw GJ, et al. Corneal lipid deposition in Cuban tree frogs (Osteopilus septentrionalis) and its relationship to serum lipids: an experimental study. J Zoo Wildl Med. 2001; 32(3):305-319.
- Wilcock BP, Njaa BL. Special Senses. In: Maxie MG, ed. Jubb, Kennedy & Palmer's Pathology of Domestic Animals. Vol 1. 6th ed. St. Louis, MO: Elsevier; 2016:407-508.
- Williams DL, Whitaker BR. The amphibian eye: a clinical review. J Zoo Wildl Med. 1994; 25(1):18-28.
- Wright K. Cholesterol, corneal lipidosis, and xanthomatosis in amphibians. Vet Clin North Am Exot Anim Pract. 2003;6(1):155-67.
- Zak A, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014; 158(2):181-188.