Signalment:
Gross Description:
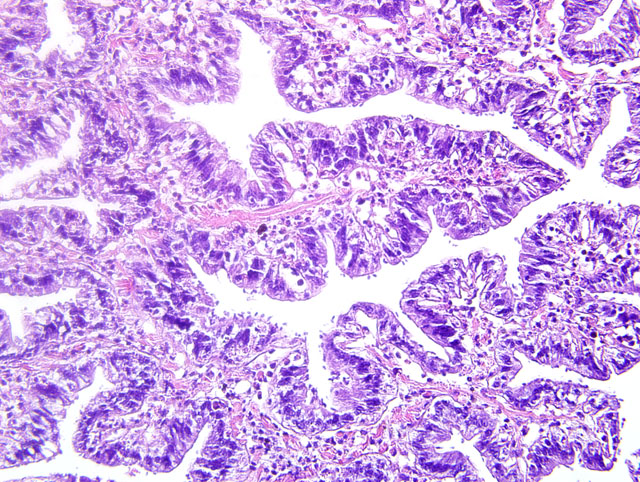
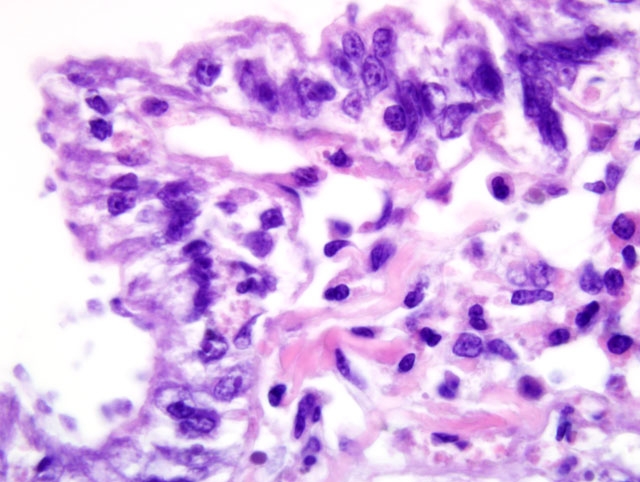
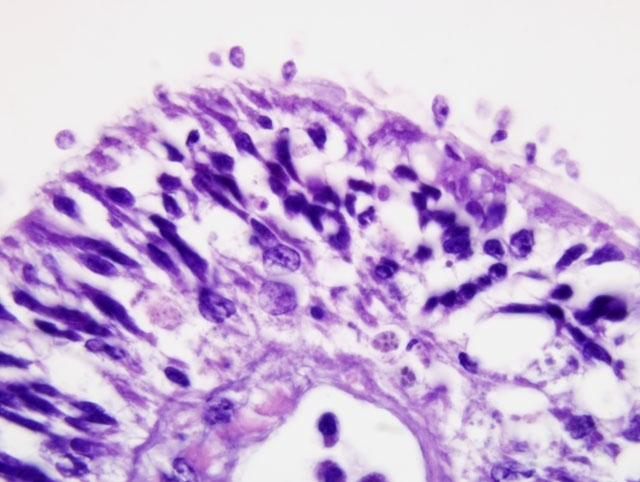
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Samples of gills tissues were processed for genomic DNA extraction, PCR amplification, and cloning and sequencing of the rRNA genes; phylogenetic analysis was conducted on the SSU, D1-D3 and D8-D10 LSU rDNA sequences. Gene sequences were deposited in GenBank. Phylogenetic analysis indicated that the Ichthyobodo species identified in this study was most closely related to those from freshwater fish.
Ultrastructural features were similarly characteristic of Ichthyobodo species. Scanning electron microscopy showed that the flagellates were flattened pyriform to trapezoidal, approximately 6-10 μm long and 3-6 μm wide. In some regions the infection was so dense that the gill surfaces were completely covered with the flagellates. Flagellates were attached to the host cells cytoplasm by a narrow cytostome via a crateriform puncture through the smooth mucous covering of the gill epithelium. Transmission electron microscopy revealed two unequal-width flagella lying in a U-shaped flagellar pocket, microtubules and striated fibers surrounding the flagellar pocket, and radiating fibres lying in a semi-circle around the cytostome primordial. The spherical nucleus had a large central nucleolus and peripheral chromatin, and the cytoplasm also contained rough endoplasmic reticulum, and mitochondria. There was an attachment plate anchoring the flagellate to the epithelial cell, and the cytostome process, reinforced with fibrillar structures, passed though the plate into the cytoplasm of the host cell.
Bodonid flagellates, of the genus Ichthyobodo, have long been recognized as significant ectoparasitic pathogens of freshwater and marine fish in aquaculture, and have been the subject of extensive research. The parasite can be found both free-swimming in the water column and attached to epithelial surfaces such as gills and skin.(6,13) The free-swimming form moves in a spiral motion with the aid of flagella. Ichthyobodo attaches to epithelial cells via a long, slender organelle that facilitates the ingestion of host cellular contents.(6,13) Ichthyobodiasis can impede osmoregulation and predispose to secondary infection; alternatively, Ichthyobodo infections are often found in association with stressful circumstances in freshwater fish.(6,13) In contrast, there are only intermittent reports of bodonids and Ichthyobodo-like flagellates from cephalopods, and their host-parasite interaction and identity have not been reported in detail.
Invertebrates, including mollusks, rely on innate immunity to combat disease, with no evidence of acquired immunity.(9) Members of the phylum Mollusca, which includes the class Cephalopoda (squids, cuttlefish, octopus and nautilus), have both cellular and humoral mechanisms of defense. Hemagluttins are the best studied and understood component of molluscan humoral immunity. These soluble factors, named after the agglutination of erythrocytes from other species, is thought to play a role in the recognition and opsonization of foreign material, although cell-free hemolymph is reported to have limited ability to agglutinate bacterial isolates in vitro.(3) The hemocyte is the primary component of cephalopod cellular immunity and develops from stem cells found in the white body, a multilobed leukopoietic organ located in the retrobulbar areas.(4) The role of octopus hemocytes in wound healing is well documented; a progression from skin wound infiltration, transformation of hemocytes to fibroblastic morphology and establishment of a dermal plug with subsequent contraction culminates in wound repair.(2) A multi-function cell with oxygen-carrying and nutrient transport capacities, the octopus hemocyte also performs phagocytosis, producing reactive oxygen species and lysozyme to destroy pathogens.(8,11) Stress and low temperature have been shown to alter the phagocytic function of hemocytes in vivo.(7) In addition to phagocytosis, hemocyte encapsulation serves as defense mechanism against foreign substances. Resident hemocytes are described in gill tissues of octopus, complicating the evaluation and characterization of potential gill pathogens.(12)
The reproductive physiology of cephalopods is interesting and enigmatic. In cephalopods including Giant Pacific octopus, endocrine secretions from the paired optic glands direct the development of senescence, characterized by loss of appetite, change in feeding behavior, loss of condition and behavioral changes that culminate in death. Hence these species of octopus have a brief lifespan of only about 3 years and inevitably die after spawning.(1) In the captive octopus of this report, it is unclear whether there is any association of senescence and enhanced susceptibility to the Ichthyobodo infections. Removal of the optic glands is reported to reduce senescenceassociated behavior and greatly extends the lifespan of cephalopods.(14)
These observations demonstrate that Ichthyobodo sp. can be a significant ectoparasitic pathogen of captive cephalopods. The host-parasite interaction and parasite ultrastructure are essentially similar to those of the better known Ichthyobodo sp. affecting teleosts. Molecular phylogeny showed that the Ichthyobodo sp. from these wildcaught captive Giant Pacific octopuses was most closely related to flagellates from several species of freshwater teleosts.
JPC Diagnosis:
Conference Comment:
Because Ichthyobodo infection is commonly associated with stress in freshwater fish, participants speculated that senescence-associated immunosuppression may have rendered this octopus particularly vulnerable to parasitism by Ichthyobodo-like flagellates. Curiously, some species of Ichthyobodo are capable of survival both in saltwater and freshwater; for instance, I. necator, which survives and causes disease over a wide range of temperatures, can survive in saltwater and cause mortality in marine-adapted salmonids. Other Ichthyobodo-like flagellates exclusively infect marine fish.(10) Ichthyobodo-like flagellate infection was reported in a laboratory cultured population of California mud-flat octopus (Octopus bimaculoides) and two related species (O. maya and O. digueti), in which the organism was initially found on the gill, then spread over the course of the disease to involve the internal surface of the mantle cavity and the body surface, leading the authors to conclude that the gill was the initial site of infection. Gross lesions included small white foci on the dorsal surfaces of the arms and mantle; however, these may have been partly attributed to co-infection with an ancistrocomid ciliate.(5)
References:
2. Bullock AM, Polglase JL, Phillips SE: The wound healing and haemocyte response in the skin of the lesser octopus Eledone cirrhosa (Mollusca: Cephalopoda) in the presence of Vibrio tubiashii. J Zool 201:185-204, 1986
3. Fisher WS, DiNuzzo AR: Agglutination of bacteria and erythrocytes by serum from six species of marine molluscs. J Invertebr Pathol 57:380-94, 1991
4. Ford LA: Host defense mechanisms of cephalopods. Annual Rev of Fish Diseases 2:25-41, 1992
5. Forsythe JW, Hanlon RT, Bullis RA, Noga EJ: Octopus bimaculoides: a marine invertebrate host for ectoparasitic protozoans. J Fish Dis 14:431-442, 1991
6. Gratzek JB: Parasites associated with freshwater tropical fishes. In: Fish Medicine, ed. Stoskopf MK, pp. 576-577. W.B. Saunders, Philadelphia, PA, 1993
7. Malham SK, Lacost A, Gelebart F, Cueff A, Poulet AS: A first insight into stress-induced neuroendocrine and immune changes in the octopus Eledone cirrhosa. Aquat Living Resource 15:187-192, 2002
8. Malham SK, Runham N, Secombes C: Lysozyme and antiprotease activity in the lesser octopus Eledone cirrhosa (Cephalopoda): developmental and comparative immunology. Immunology 22:22-37
9. Mydlarz LD, Jones LE, Harvell CD: Innate immunity, environmental drivers, and disease ecology of marine and freshwater invertebrates. Annu Rev Ecol Evol Syst 37:251-288, 2006
10. Noga EJ: Fish Disease: Diagnosis and Treatment, pp. 108-110. Iowa State University Press, Ames, IA, 2000
11. Rodriguez-Dominguez H, Soto-Bua M, Iglesias-Blance R, Crespo-Gonzalez C, Arias-Fernandez C, Garcia- Estevez J: Preliminary study on the phagocytic ability of Octopus vulgaris Cuvier, 1797 (Mollusca: Cephalopoda) haemocytes in vitro. Aquaculture 254:563-570, 2006
12. Schipp R, Mollenhauer S, von Boletzky S: Electron microscopical and histochemical studies of differentiation and function of the cephalopod gill (Sepia officinalis L.) Zoomorphology 93:193-207, 1979
13. Thune RL: Parasites of catfishes. In: Fish Medicine, ed. Stoskopf MK, pp. 528-529. W.B. Saunders, Philadelphia, PA, 1993
14. Wodinsky J: Hormonal inhibition of feeding and death in octopus: control by optic gland secretion. Science 198:948-951, 1977