Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 2
August 30th, 2017
CASE III: 14-1424 (JPC 4066348).
Signalment: 16-year-old, female, Hafflinger horse (Equus caballus).
History: The animal was presented in emergency care center for acute neurological disorders (unsteadiness, fallings and decubitus, amaurosis). Despite critical care, development of a semi-comatose state and convulsions led to euthanasia.
Gross Pathology: At necropsy, the liver had an increased consistency with a variegated aspect and somewhat bulging tissue at section. The brain did not show visible changes.
Laboratory results: Blood analyses showed severe neutrophilic leukocytosis (Leukocytes 32,99.109/L, neutrophils 28,5.109/L, lymphocytes 2,67.109/L).
Biochemistry exam showed hyperproteinemia (80 g/L), hypoalbuminemia (25 g/L) and hyperglobulinemia (54 g/L).
A severe augmentation of gamma-GT was present (510 U/L), and increased values for GLDH (11,7 UI/L), total bilirubin (89 µmol/L), CK (> 2036 U/L), and lactates (> 12 mmol/L).
CSF fluid analysis did not show significant changes.
PCR (blood for Babesia sp., Theileria sp., and blood and CSF for Borrelia sp.) were negative.
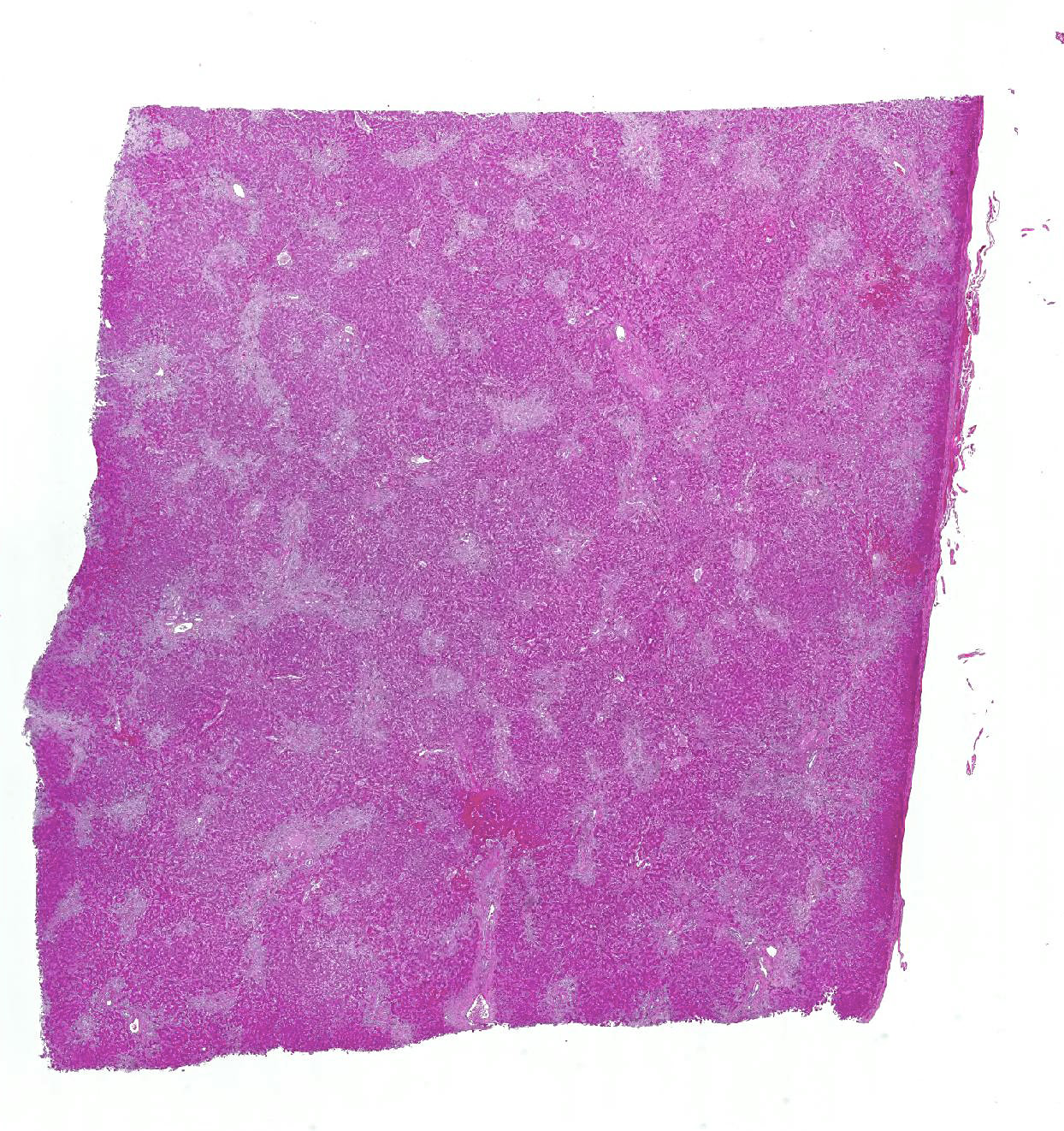
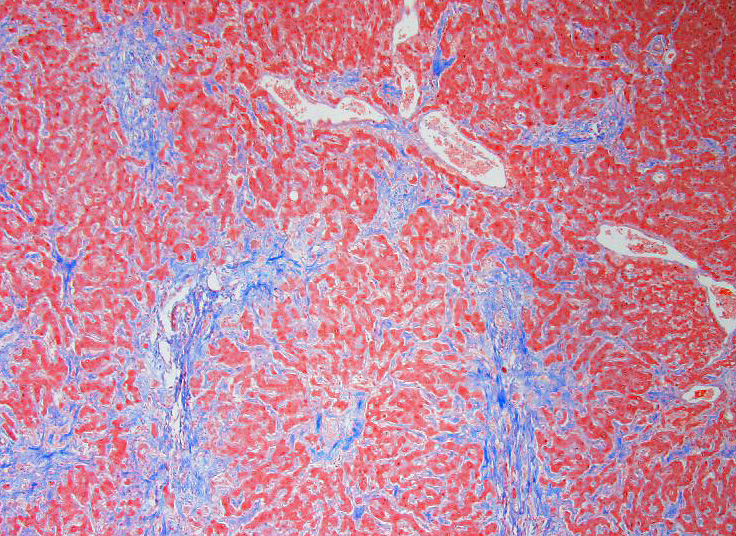
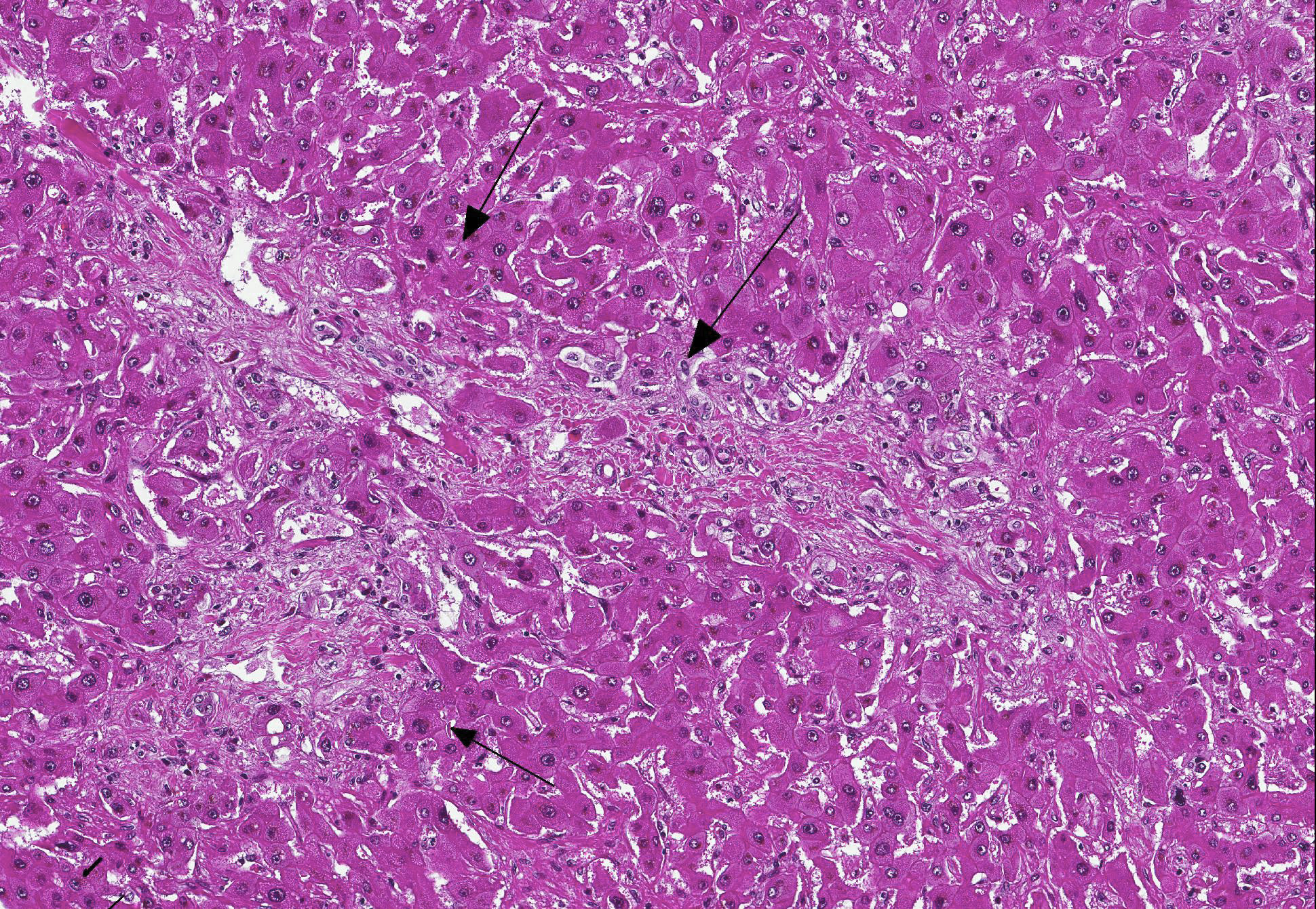
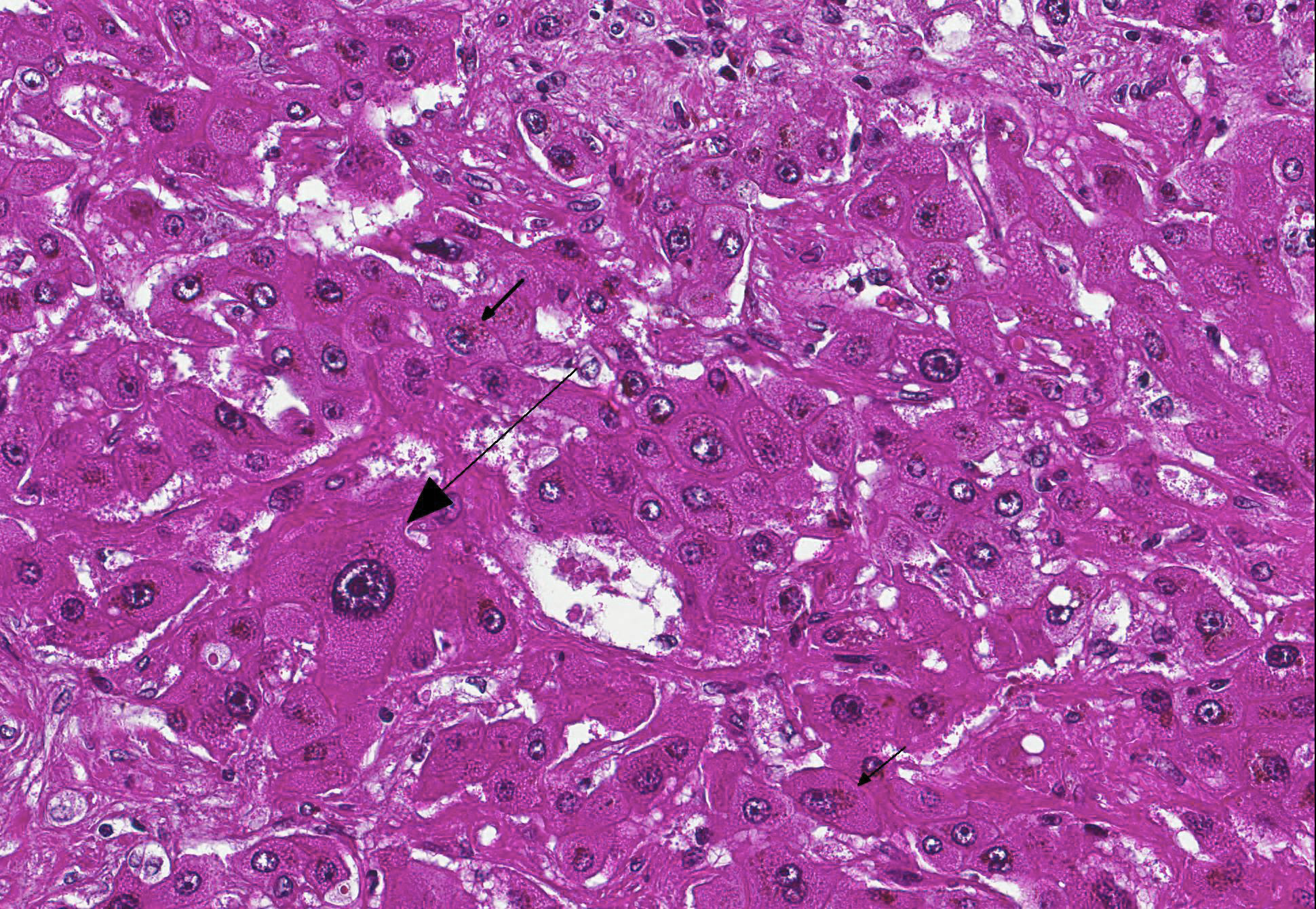
Microscopic Description: Liver: Diffusely, there is marked fibrosis, mostly restricted to portal tract, and multifocal bridging between these portal tracts, distorting normal hepatic architecture. Isolation of individual hepatocytes by fibrosis is also present at the edges of the lobules. Diffusely, there are hepatocytes which are moderately to severely enlarged, with swollen nuclei. Cytoplasm is also enlarged and is vacuolated. In several portal tracts, bile duct proliferation is present. Multifocally, little hemorrhages and discrete infiltration of neutrophils, lymphocytes and macrophages are present.
Contributors Morphologic Diagnosis: Liver: Hepatocellular degeneration, diffuse, marked, with megalocytosis. Generalized portal and bridging fibrosis and moderate bile duct proliferation, Haflinger, equine.
Contributors Comment: Such changes observed in the liver are suggestive of pyrrolizidine alkaloids intoxication. These alkaloids are toxic to the liver, leading to irreversible lesions when intoxication comes to chronicity, especially in pigs, horses and cattle. These toxic alkaloids are not directly toxic, and necessitate bioactivation in hepatocytes (especially those in centrilobular region), leading to binding of these agents to proteins and nucleic acids. It results then in inhibition of mitosis, without inhibiting DNA synthesis, leading to megalocytosis. Associated to megalocytosis, there is fibroplasia and bile duct proliferation (fibroplasia is marked in cattle, moderate in horses and often minimal in sheep). Pyrrolizidine alkaloids are not the only toxic substances to cause such megalocytosis. Indeed, aflatoxins and nitrosamines can lead to this change in liver.1,2
Clinically, chronic intoxication by pyrrolizidine alkaloids is characterized by liver failure and its possible consequences (icterus and photosensitization). Secondary neurological signs can develop, known as hepatic encephalopathy. Histopathological analysis of the brain of this horse showed presence of Alzheimer type II cells, consistent with this syndrome. In species other than the horse, spongiosis is also present in addition to Alzheimer type II cells.3
Many plants containing pyrrolizidine alkaloids can be ingested (Senecio spp., Crotalaria spp., Heliotropium spp.). In this particular case, the plant responsible for these lesions was not identified; given the wide distribution of Senecio vulgaris in the region where the horse lived, its consumption was very likely the origin of this chronic intoxication.4
JPC Diagnosis: Liver: Fibrosis, portal and bridging, diffuse, moderate with hepatocellular loss, karyomegaly and megalocytosis, and biliary hyperplasia, Haflinger, equine.
Conference Comment: This case provides a classic example of pyrrolizidine alkaloid toxicity in the liver. Participants described the dense bridging fibrosis from periportal regions that surrounds and separates hepatocytes and effaces the limiting plate as well as the ductular reaction with cholestasis. Occasionally obliterated centrilobular veins were suggestive of veno-occlusive disease. Particularly prominent are the perinuclear accumulations of bile pigment within hepatocytes and multifocal karymegalic and/or multinucleated hepatocytes which are a characteristic finding in several varieties of toxic hepatic diseases.
The contributor offers a concise review of pyrrolizzidine alkaloid toxicity including pathogenesis and clinical signs. The submitted blood work was reviewed during the conference, illustrating the degree of cholestasis and hepatocellular damage (elevated GGT and bilirubin) as well as the musculoskeletal damage and reversion to anaerobic metabolism due to persistent convulsions (elevated CK and lactate).
The moderator led a brief discussion of hepatic encephalopathy caused by hyperammonemia. Within the large intestine, breakdown of protein and urea by microflora occurs routinely to produce ammonia. Ammonia is also produced within the liver (hepatic deamination of amino acids) and in peripheral tissues (from metabolism of glutamate). Normally, ammonia is removed the first time through the liver via portal circulation, whereupon it enters the urea cycle. However, acute hepatic disease can result in buildup of ammonia within the circulation which passes through the blood-brain barrier, and causes a decrease in energy metabolism, astrocyte injury and edema formation, and neuronal injury. Overworked, damaged astrocytes cluster together in pairs with enlarged swollen nuclei, margination of chromatin, and prominent nucleoli to form Alzheimer type II astrocytes.3
Various plant species that produce different types of alkaloids were reviewed: Compositae (Senecio spp.), Leguminosae (Crotalaria spp., Tephrosia spp.), and Boranginaceae (Heliotropium, Cynoglossum, Amsinckia, Echium, Trichodesma and Symphytum spp.). Croatalaria sp. affects the widest range of tissues. Pyrrolizidine alkaloid toxicity depends on four factors: which alkaloids are produced, which organ is affected and the metabolic activity of target cells, the rate the alkaloid is converted to toxin versus the efficiency of glutathione conjugation, and the species, sex, and age of the animal. Monogastric species are the more susceptible to toxicity, as they lack a rumenal degradative pathways for toxin; sheep and goats have the greatest resistance. Horses are more likely to develop hepatic encephalopathy which causes head-pressing and compulsive walking and leads to idiomatic names like walkabout and walking disease.3
Several differential diagnoses for hepatotoxins were discussed during the conference, including aflatoxins, nitrosamines, triterpenes, methylazoxymethanol, and indospicine.
|
Toxin |
Produced by |
Microscopic changes |
|
Aflatoxin (B1 most potent) |
Aspergillus flavus, A. parasiticus, Penicillium puberulum |
Biliary hyperplasia and hemorrhagic necrosis; megalocytosis less prominent |
|
Nitrosamines (dimethylnitrosamine) |
Reaction product of trimethylamine with sodium nitrite (preservative) in herring meal |
Not specific slowly developing hepatotoxicity; megalocytosis, fatty change, bile accumulation |
|
Triterpenes (predominately Lantadene A and C) |
Lantana camara, an ornamental shrub native to the Americas and Africa |
Focal hepatic necrosis, canalicular cholestasis; icterus and photosensitization |
|
Methylazoxymethanol |
Cycas or Zamiaceae spp. plants produce toxin |
Centrilobular necrosis Cycads also produce neurotoxic animo acid ?-N-methylamino-L-alanine (BMAA) cause CNS lesions due to excitotoxicity |
|
Indospicine (6-amidino-2-hexanoic acid) |
Legumes of the genus Indigofera |
Cattle/dogs: centrilobular necrosis, cholestasis, hepatic encephalopathy Horses: neurologic disorder Birdsville horse disease |
Chart adapted from Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier; 2016:330-343.
Species differences with regard to target tissues and histologic lesions were also discussed. Cattle tend to develop more severe fibrosis that can lead to veno-occlusion. Sheep affected by Heliotropium europaeum and Echium plantagineum may develop severe intravascular hemolysis if liver copper content is high and hepatic mass is decreased. In pigs, pyrrolizidine alkaloids cause pulmonary emphysema with diffuse fibrosis and potential renal insufficiency. Experimental exposure in rats leads to progressive pulmonary disease, pulmonary hypertension, and cor pulmonale with necrotizing vasculitis of the pulmonary arterioles. Lastly, goats are relatively resistant, but with exceptionally high doses of Croatalaria retusa they develop acute lesions consisting of centrilobular hemorrhagic necrosis, midzonal hepatocyte swelling and vacuolation.
Finally, a review of photosensitization was provided by the moderator. Photosensitization is the inflammation of unpigmented skin (usually) due to the reaction of ultraviolet light of wavelengths 290-400 nm on photodynamic compounds that have become bound to dermal cells. Type I or primary photosensitization those photodynamic compounds were deposited unchanged in the skin before the liver (healthy) could excrete it. Examples were given of photosensitizing plants that contain pigments from the helianthrone (St. Johns Wort, buckwheat) or furocoumarin (spring parsley, bishops weed, Dutchmans breeches, giant hogweed) family. The pigments produced by helianthrone are hypericin and fagopyrin, and furocoumarin are psoralens.
Type II photosensitization is due to defective pigment synthesis associated with several congenital conditions is specific breeds. Bovine congenital hepatopoietic porphyria (pink tooth) is most common in Shorthorn, Ayrshire, Holstein and Jamaican cattle and causes a deficiency in uroporphyrinogen III cosynthetase which results in red-brown coloration of porphyrin in dentin and bone and skin lesions due to accumulated uroporphyrins which absorb UVA radiation. Siamese cats are also prone to congenital photosensitization and the deficiency is also thought to be uroporphyrinogen III cosynthetase. Another inherited deficiency is seen in Limousin cattle that develop bovine erythropoietic protoporphyria due to ferrochelatase deficiency with leads to protoporphyrin IX accumulation in blood and tissue. In this disease, photodermatitis is the only lesion. Type III (hepatogenous) photosensitization is the most common form and usually accompanies cholestasis of more than a few days duration in herbivores eating green feed that are kept in direct sunlight. Phytoporphyrin (phylloerythrin), a porphyrin produced in herbivores by rumenal microflora as a breakdown product of chlorophyll, is released into portal circulation, removed by hepatocytes, and excreted in bile. Cholestasis increases retention of phytoporphyrin in the blood and result in dermatitis of unpigmented areas of skin exposed to sunlight.3
Contributing Institution:
Anatomie Pathologique
Vetagro sup
Campus vétérinaire
References:
1. Cortinovis C, Caloni F. Epidemiology of intoxication of domestic animals by plants in Europe, The Veterinary Journal. 2013; 197(2):163-168.
2. Cullen JM, Brown DL. Hepatobiliary system and exocrine pancreas. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby; 2007:439-440.
3. Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier; 2016:291-292, 294, 330-343.
4. Stalker MJ, Hayes MA. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 5th ed. Philadelphia, PA: Elsevier; 2007:373-374.
5. Zachary JF: Nervous system. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby; 2007:814-815.