Wednesday Slide Conference, Conference 14, Case 3
Signalment:
6-week-old, crossbred pig, Sus scrofa domesticus
History:
Pig from an experimental inoculation study.
Microscopic Description:
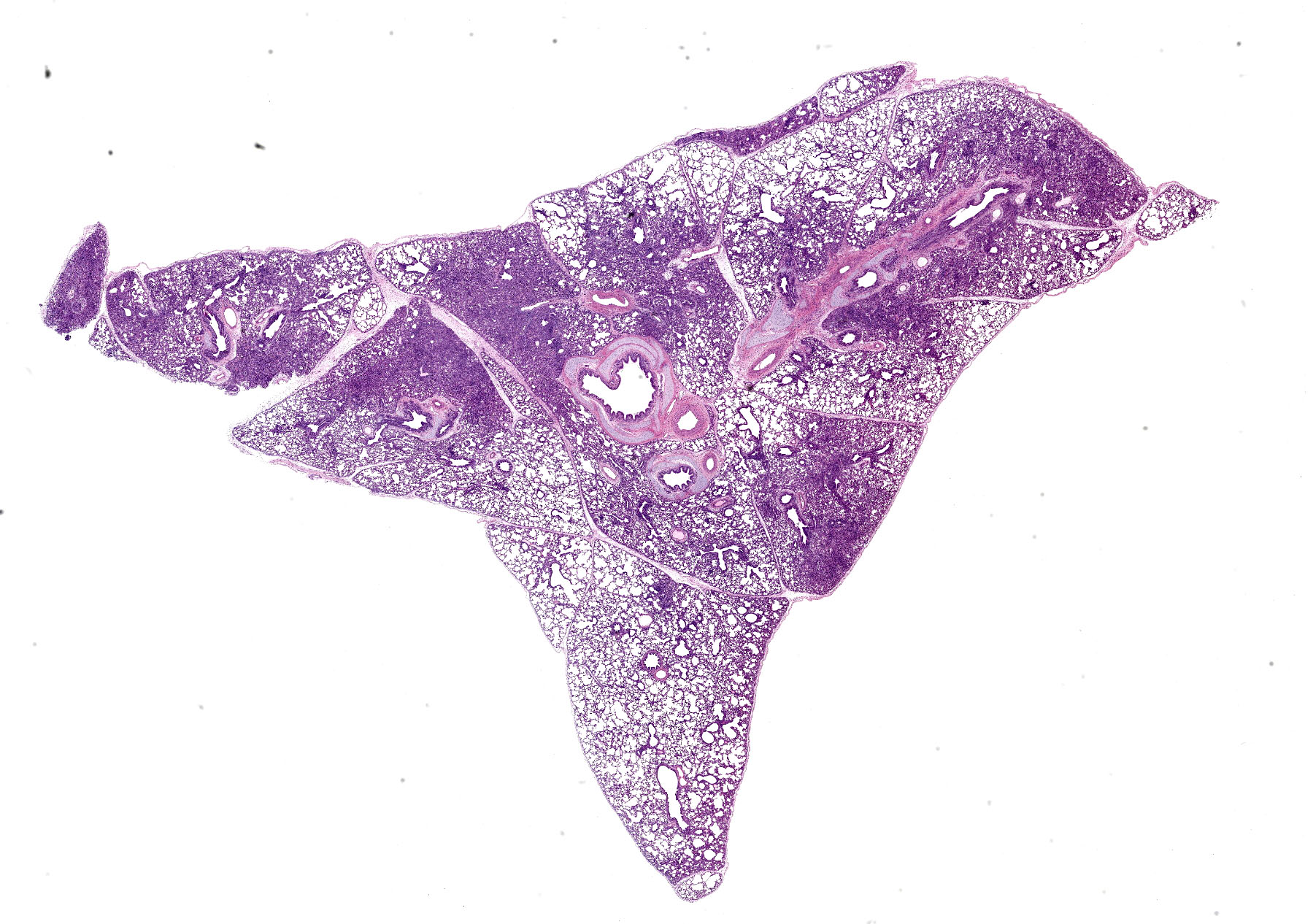
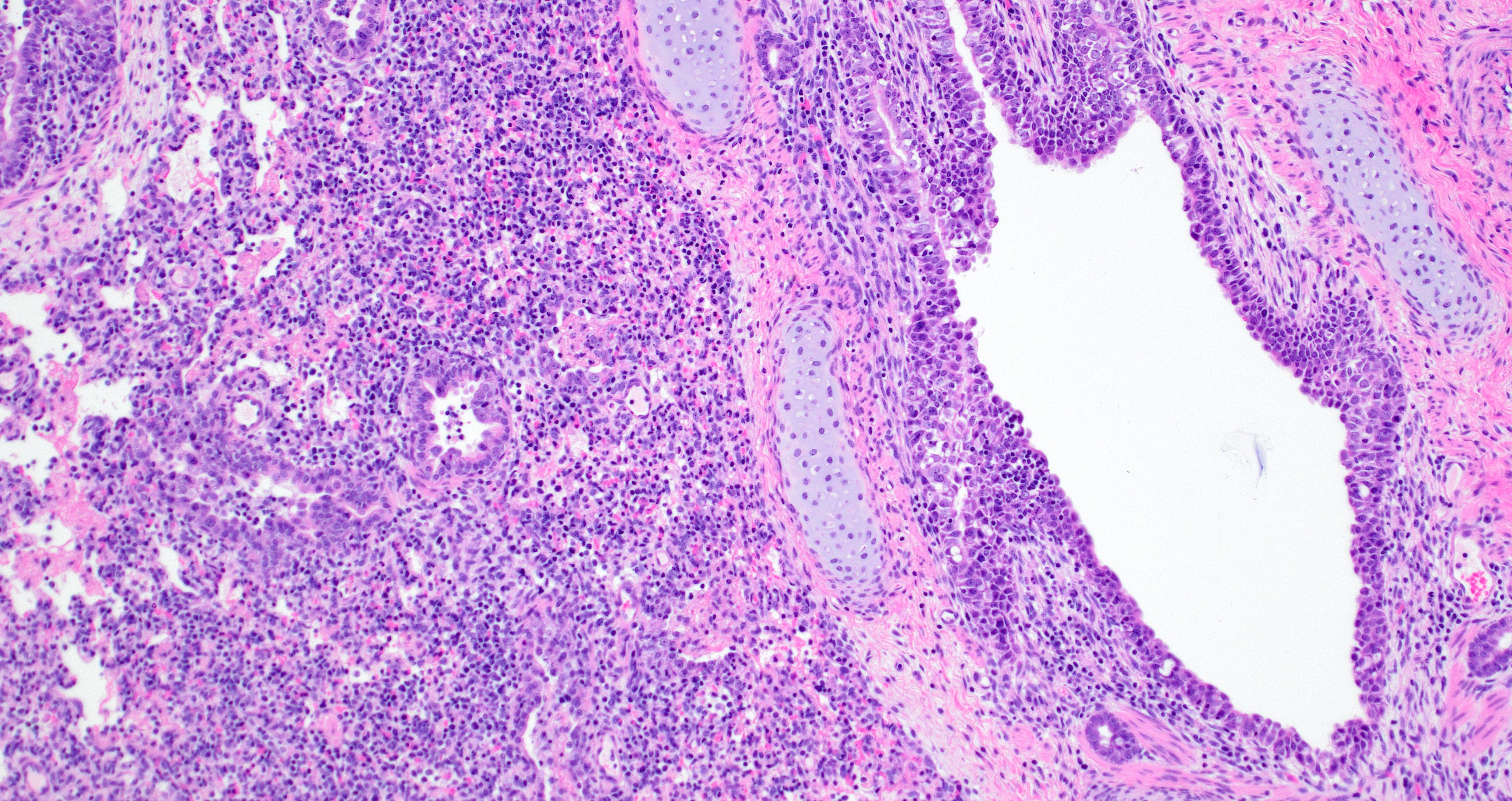
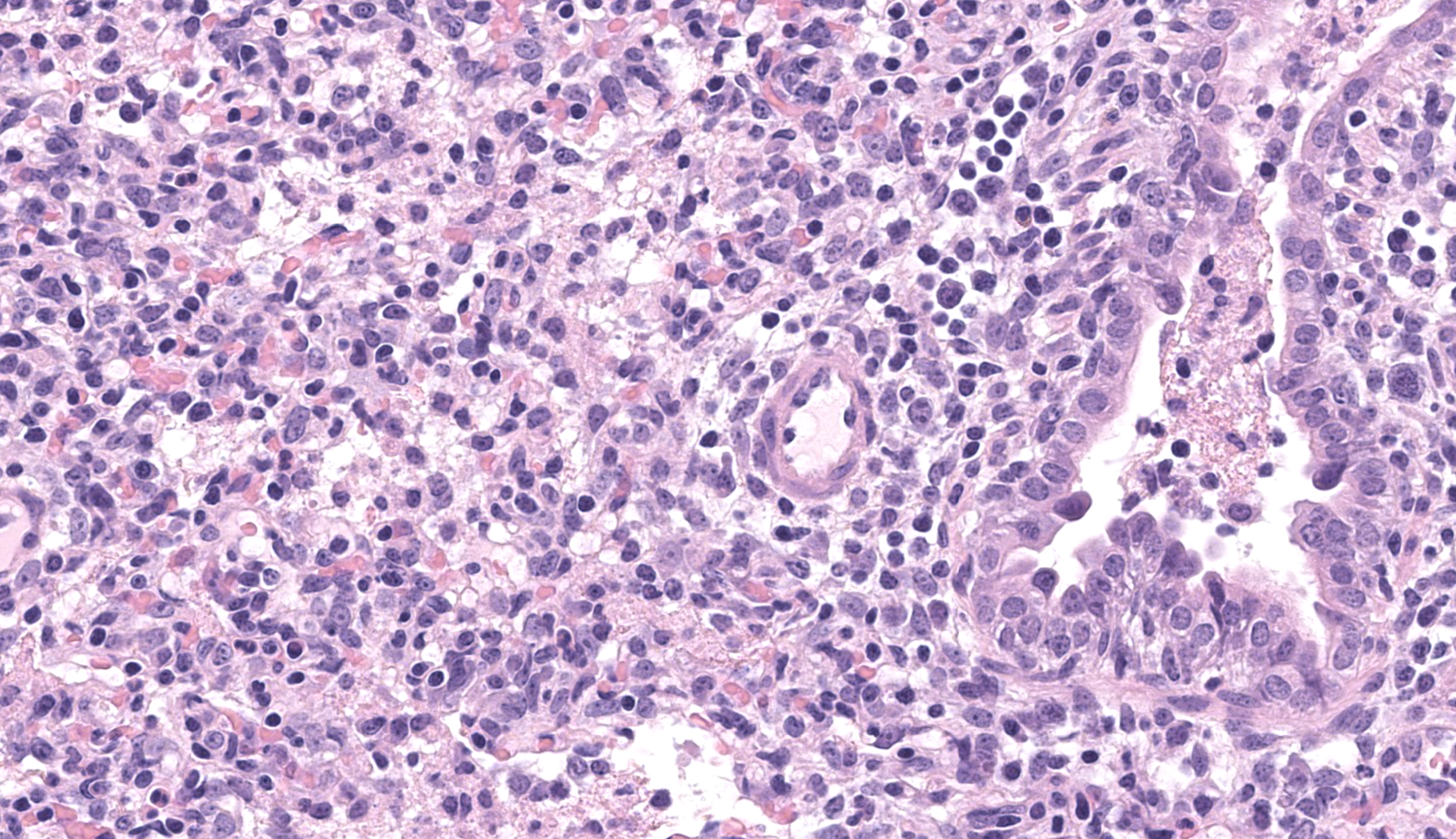
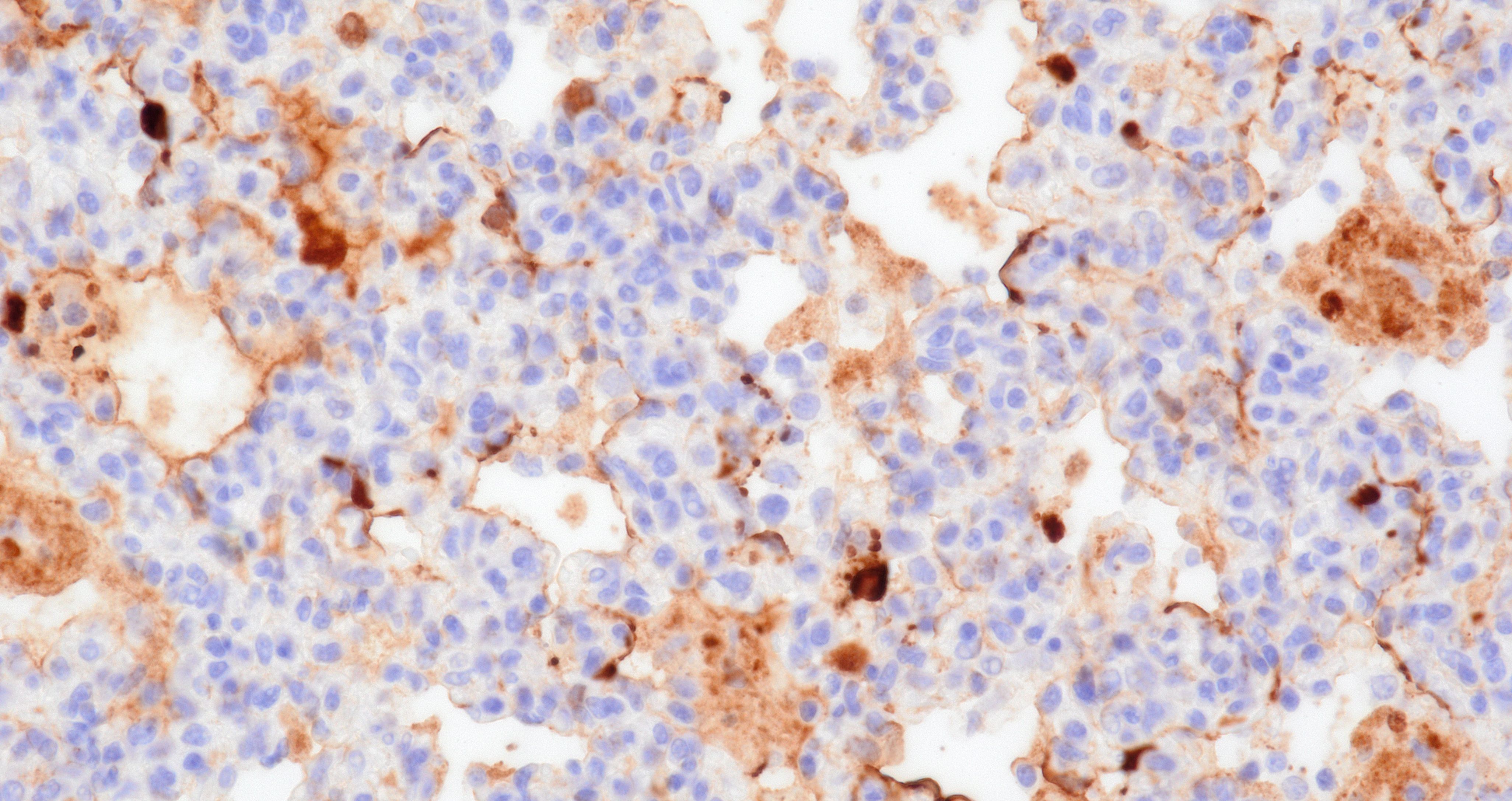
Lung. Section of lung in which there is minimal to mild dilation of bronchi and bronchioles. These airways are empty or partially filled by fibrin, neutrophils, cell debris and macrophages. The airway epithelium is segmentally eroded in multifocal areas and small amounts of cell debris cover these regions. Other bronchi and bronchioles are lined by hypertrophied epithelial cells with large, non-basilar nuclei and occasional mitotic figures (epithelial regeneration). In these conducting airways, there are intraepithelial lymphocytes and neutrophils. The lamina propria of some bronchi have minimal to mild infiltrates of lymphocytes and plasma cells. Some bronchi, bronchioles, small arteries, and veins have mild to moderate adventitial infiltrates of lymphocytes and plasma cells that occasionally form nodular aggregates. There is multifocal minimal to moderate thickening of alveolar septae commonly around airways due to mild multifocal hyperplasia of type II cells and infiltrates of lymphocytes and macrophages. The alveolar lumens of multiple lobules contain seroproteinaceous fluid, cell debris, fibrin, occasional macrophages, and neutrophils. The interlobular septae are expanded by clear space (edema) and a mixed inflammatory infiltrate composed predominately of lymphocytes, macrophages, and plasma cells.
Contributor’s Morphologic Diagnosis:
Lung: Mild to moderate multifocal acute necrotizing bronchitis and bronchiolitis with moderate to marked peribronchiolar and peribronchiolar interstitial pneumonia
Contributor’s Comment:
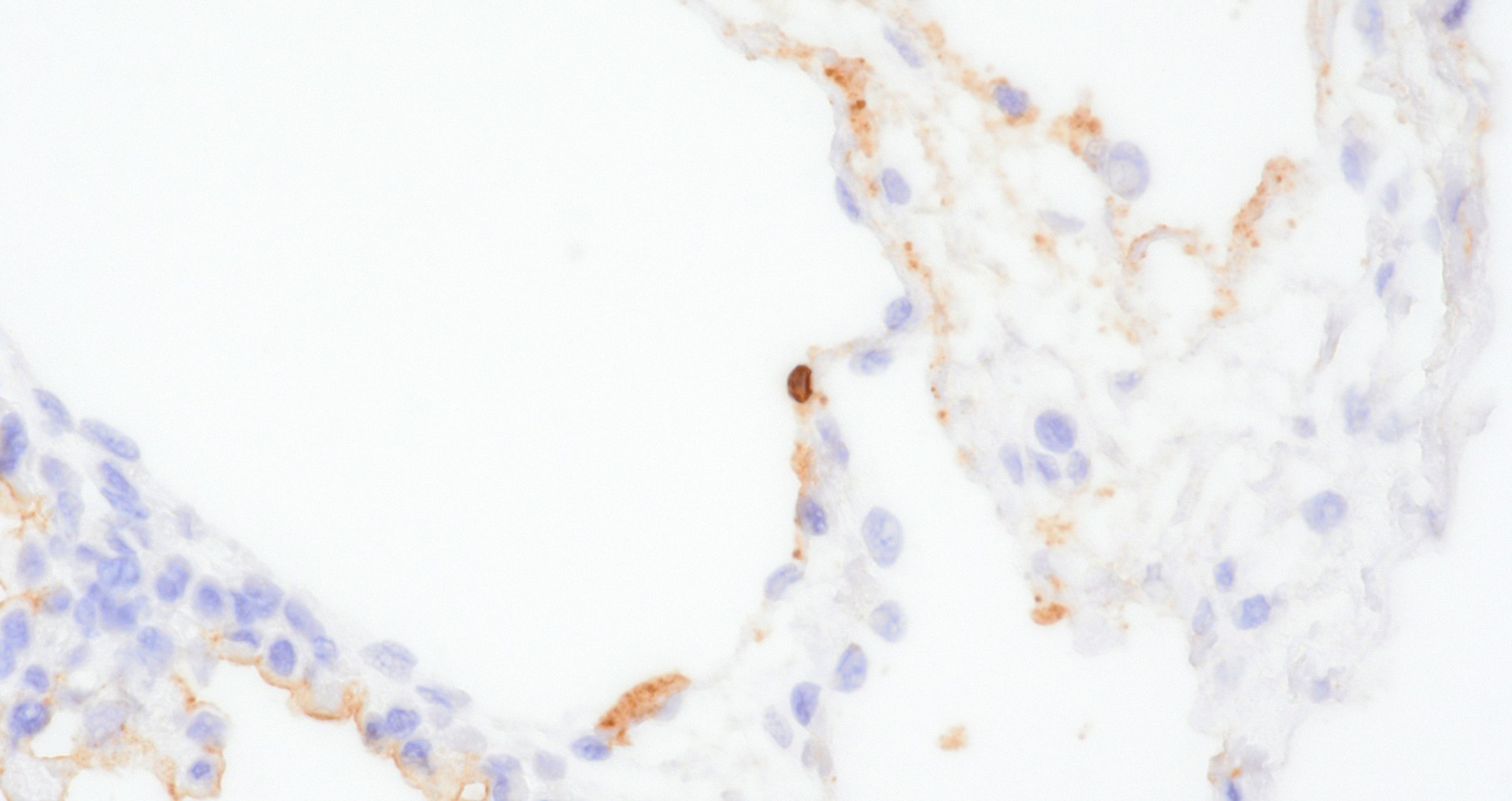
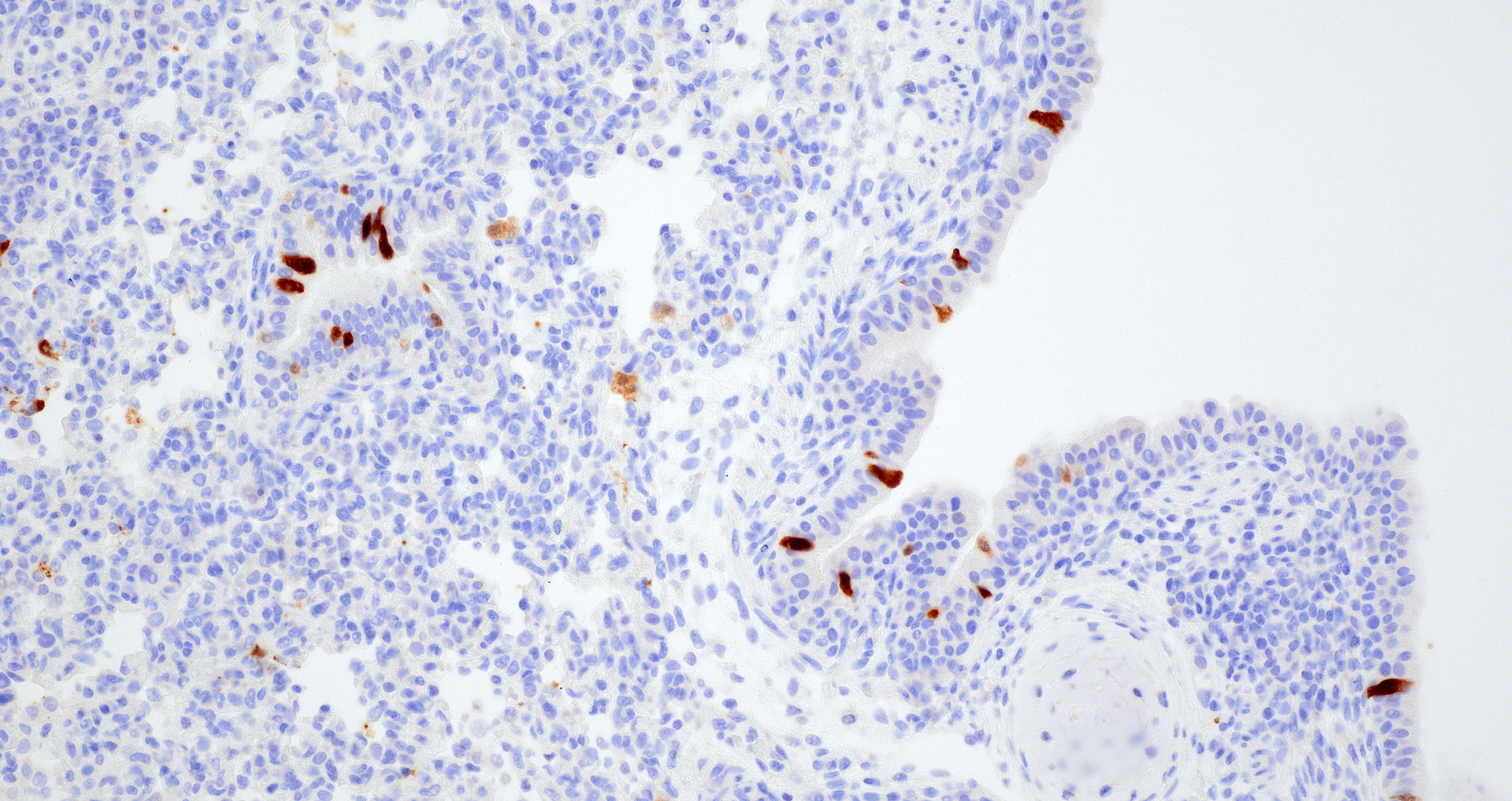
The lung in this case featured macroscopic lesions consistent with influenza A virus infection and was from a pig experimentally inoculated with a highly pathogenic strain of avian influenza (HPAI; A/bald eagle/Florida/W22-134-OP/2022; Genotype B1.1) belonging to the goose/Guangdong 2.3.4.4b hemagglutinin phylogenetic clade. This clade has caused mass mortality events in avian and mammalian species across the globe since 2022.2,3,5,15 The transcontinental circulation of clade 2.3.4.4b viruses within bird populations allows reassortment with low pathogenicity avian influenza (LPAI) viruses resulting in numerous genotypes of different phenotypes across species and the globe, some of which caused neurologic disease in mammals, a manifestation not observed in our study.6,7,20 Nonetheless, the presence of NP (nucleoprotein) antigen in endothelial cells of pigs infected with A/bald eagle/FL/22 suggest this strain may spread systemically.
A subset of mammalian isolates of HPAI H5N1 clade 2.3.4.4b acquired mammalian adaptation markers (E627K, D701N, or T271A) in the polymerase basic (PB) 2 protein that pose a public health risk should these viruses gain efficient transmission in mammals.4,10 Mammalian adaption of HPAI is multigenic, and the genetic changes necessary for H5N1 strains to adapt to and transmit in swine are not well understood. Although uncommon, incursion of LPAI into commercial swine herds in North America occurs often due to unidentified sources.8,9,11 Swine-adapted IAV (influenza A viruses) have a propensity for evolution through polymerase errors and reassortment followed by transmission through populations of densely housed commercial pigs and live pig transport. If an avian IAV strain such as H5Nx 2.3.4.4b were to infect domestic swine, there is potential for interspecies transmission, reassortment with endemic swine IAV, and/or acquisition of adaptive mutations that may allow an avian-to-mammalian switch.10 Increased viral fitness leading to transmission of LPAI strains following reassortment with swine adapted IAV in pigs was demonstrated both in commercial swine herds and experimentally.1,11 Furthermore, on farm transmission between pigs of a HPAI H5N1 virus and identification of a purified clone that recognizes α2,6 sialic acid receptors was reported.14 More recently, infection of domestic pigs with clade 2.3.4.4b was demonstrated.16
Swine are commonly identified as a ‘mixing vessel’ supporting reassortment that could lead to antigenic shift.10 Yet, at a receptor level, it has been suggested that swine are no more susceptible to infection by avian IAVs than humans.10 The HA (hemagglutinin) of HPAI 2.3.4.4b H5N1 preferentially bind to α2,3-linked sialic acids on host cells,7 which are at low abundance in the upper respiratory tract of swine.12 This low abundance and historic lack of PB2 mammalian adaption mutations may explain why avian IAV does not readily transmit among swine.
The quantity of α2,3-linked sialic acids is relatively higher in the lower respiratory tract of pigs and humans and localizes to pneumocytes and non-ciliated bronchiolar cells.13,17,19 This distribution is consistent with the extent and distribution of IAV nucleoprotein and RNA labeling in the lung of pigs inoculated with A/bald eagle/FL/22. Labeling is not commonly observed in swine-adapted IAV infection which is most commonly restricted to the conducting airway epithelium. Host-adapted IAV most consistently infect airway epithelium lining conducting airways, resulting in epithelial cell degeneration and necrosis that leads to loss of the epithelial integrity, triggering airway and alveolar inflammation that alters function and interferes with gaseous exchange. Bronchitis and bronchiolitis were a feature of this case due to replication of A/bald eagle/FL/22 in the respiratory epithelium. However, the prominent accumulation of alveolar luminal exudate because of pneumocyte infection and necrosis is not commonly observed in swine-adapted IAV infection and is likely in part due to the distribution of α2,3-linked sialic acids in swine and propensity of 2.3.4.4b H5N1 to bind α2,3-linked sialic acids.
There is a risk of reassortment of the HPAI H5N1 2.3.4.4b lineage with endemic swine IAV based on the susceptibility of swine to this lineage, the prevalence of IAV infection and co-morbidities in swine herds, and animal husbandry practices.18,21 Continued phenotypic assessment of HPAI H5NX 2.3.4.4b strains will facilitate awareness and detection capabilities in the swine sector and inform risk assessments and warning systems to safeguard human health.
Contributing Institution:
Mark R. Ackermann, DVM, PhD, DACVP
Center Director
USDA/ARS-National Animal Disease Center
1920 Dayton Avenue
Ames, Iowa 5001
JPC Diagnosis:
Lung: Pneumonia, bronchointerstitial, lymphohistiocytic, subacute, multifocal to coalescing, moderate.
JPC Comment:
The contributor provides a detailed summary of HPAI that remains quite relevant to ongoing events several years after this experimental case was first sent to us. Dr. Brown discussed the current paradigm of dairy cattle-associated HPAI with the group. Recent research on the distribution of α2,3-linked sialic acids has demonstrated that the bovine mammary gland (in addition the respiratory tract) contains abundant receptors for avian influenza viruses.22 Replication of the virus within the mammary gland epithelium is characterized by attenuation of secretory alveoli and ducts with intraluminal neutrophilic exudate, though the degree of mastitis is moderate.22 HPAI-positive milk has been to be infectious when given orally to mice;23 replication competent wild rodents may also play a role in the continued spread of HPAI on cattle farms.24
The survival and subsequent infectivity of HPAI in milk presents an important public health question.22,23,24 Simple assays injecting virus into milk samples (‘spiking’) have demonstrated the hardiness of influenza A virus to heat treatment; the virus also remains infective in raw milk samples stored at refrigeration temperatures for 5 weeks.23 Natural infection with replication in the mammary gland likely makes inactivation of the virus more difficult due to partial incorporation with fat globules and casein protein which act as an additional barrier to heat treatment.23 That stated, the effectiveness of conventional commercial pasteurization of milk in addressing influenza A is still being characterized. In our experience (Army Veterinary Services), many commercial facilities have adapted additional time and/or temperature controls for milk, yogurt, and other dairy products to achieve a desired product composition and provide a wide safety buffer. The recommendation to avoid raw milk nonetheless remains prudent.
The gross pathology-histology correlate was interesting for this case. We differed slightly in our interpretation of the predominant inflammatory cell type for this pneumonia and felt that the degree of necrosis was overshadowed by predominantly lymphocytes and histiocytes. Several conference participants also noted bronchial and glandular epithelial regeneration which hinted at a slightly longer time course as well. Select alveolar macrophages contained irregular eosinophilic material that some participants interpreted as botryoid inclusions (i.e. from porcine circovirus) while others interpreted necrotic macrophages as a potential correlate of PRRSV which Dr. Brown acknowledged. Absent the history provided in this case, these two viruses are important differentials in porcine granulomatous pneumonias.
References:
- Abente EJ, Kitikoon P, Lager KM, Gauger PC, Anderson TK, Vincent AL. A highly pathogenic avian-derived influenza virus H5N1 with 2009 pandemic H1N1 internal genes demonstrates increased replication and transmission in pigs. J Gen Virol. 2017;98: 18-30.
- Agriculture USDo: 2022-2023 Confirmations of Highly Pathogenic Avian Influenza in Commercial and Backyard Flocks. 2023.
- Agriculture USDo: 2022-2023 Detections of Highly Pathogenic Avian Influenza in Mammals. 2023.
- Authority EFS, Prevention ECfD, Control, et al. Avian influenza overview December 2022 – March 2023. EFSA Journal. 2023;21: e07917.
- Bevins SN, Shriner SA, Cumbee JC, Jr., et al. Intercontinental Movement of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4 Virus to the United States, 2021. Emerg Infect Dis. 2022;28: 1006-1011.
- Graaf A, Piesche R, Sehl-Ewert J, et al. Low Susceptibility of Pigs against Experimental Infection with HPAI Virus H5N1 Clade 2.3.4.4b. Emerg Infect Dis. 2023;29: 1492-1495.
- Kandeil A, Patton C, Jones JC, et al. Rapid evolution of A(H5N1) influenza viruses after intercontinental spread to North America. Nat Commun. 2023;14: 3082.
- Karasin AI, Brown IH, Carman S, Olsen CW. Isolation and characterization of H4N6 avian influenza viruses from pigs with pneumonia in Canada. J Virol. 2000;74: 9322-9327.
- Karasin AI, West K, Carman S, Olsen CW. Characterization of avian H3N3 and H1N1 influenza A viruses isolated from pigs in Canada. J Clin Microbiol. 2004;42: 4349-4354.
- Long JS, Mistry B, Haslam SM, Barclay WS. Host and viral determinants of influenza A virus species specificity. Nat Rev Microbiol. 2019;17: 67-81.
- Ma W, Vincent AL, Gramer MR, et al. Identification of H2N3 influenza A viruses from swine in the United States. Proc Natl Acad Sci U S A. 2007;104: 20949-20954.
- Neumann G. H5N1 influenza virulence, pathogenicity and transmissibility: what do we know? Future Virol. 2015;10: 971-980.
- Nicholls JM, Chan MC, Chan WY, et al. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat Med. 2007;13: 147-149.
- Nidom CA, Takano R, Yamada S, et al. Influenza A (H5N1) viruses from pigs, Indonesia. Emerg Infect Dis. 2010;16: 1515-1523.
- Prevention CDCa: Technical Report: Highly Pathogenic Avian Influenza A(H5N1) Viruses. 2023
- Rosone F, Bonfante F, Sala MG, et al. Seroconversion of a Swine Herd in a Free-Range Rural Multi-Species Farm against HPAI H5N1 2.3.4.4b Clade Virus. Microorganisms. 2023;11: 1162.
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440: 435-436.
- Trevisan G, Schwartz KJ, Burrough ER, et al. Visualization and application of disease diagnosis codes for population health management using porcine diseases as a model. Journal of Veterinary Diagnostic Investigation. 2021;33: 428-438.
- Yao L, Korteweg C, Hsueh W, Gu J. Avian influenza receptor expression in H5N1-infected and noninfected human tissues. Faseb j. 2008;22: 733-740.
- Youk S, Torchetti MK, Lantz K, et al. H5N1 highly pathogenic avian influenza clade 2.3.4.4b in wild and domestic birds: Introductions into the United States and reassortments, December 2021-April 2022. Virology. 2023;587: 109860.
- Zeller MA, Anderson TK, Walia RW, Vincent AL, Gauger PC. ISU FLUture: a veterinary diagnostic laboratory web-based platform to monitor the temporal genetic patterns of Influenza A virus in swine. BMC Bioinformatics. 2018;19: 397.
- Nelli RK, Harm TA, Siepker C, et al. Sialic Acid Receptor Specificity in Mammary Gland of Dairy Cattle Infected with Highly Pathogenic Avian Influenza A(H5N1) Virus. Emerg Infect Dis. 2024 Jul;30(7):1361-1373.
- Guan L, Eisfeld AJ, Pattinson D, et al. Cow's Milk Containing Avian Influenza A(H5N1) Virus - Heat Inactivation and Infectivity in Mice. N Engl J Med. 2024 Jul 4;391(1):87-90.
- Usui T, Uno Y, Tanaka K, Tanikawa T, Yamaguchi T. Susceptibility of Synanthropic Rodents (Mus musculus, Rattus norvegicus and Rattus rattus) to H5N1 Subtype High Pathogenicity Avian Influenza Viruses. Pathogens. 2024 Sep 5;13(9):764.