Signalment:
12-year-old, male, intact Rhesus macaque (
Macaca mulatta).This monkey was inoculated with SIVmac251 and had undergone routine phlebotomies. Several months after inoculation, the animal developed diarrhea and loss of appetite. The animal was treated with Baytril-�, with partial resolution of the animals diarrhea and improvement in appetite. Following discontinuation of Baytril-�, the overall condition of the animal continued to decline with increased respiratory effort at rest and mucoid nasal discharge. Complete blood count and blood chemistry were within normal limits. A weight loss of 5 kilograms (from 21 to 16 kilograms) was recorded over a three month period. At the end of this period, the animal still had marked diarrhea and pneumonia. A cardiac ultrasound at this time revealed a thrombus attached to the right atrioventricular valve. The animal was placed on amoxicillin treatment; however, due to failure to respond to treatment, the animal was euthanized.
Gross Description:
The animal was markedly obese. The axillary and inguinal lymph nodes were mildly enlarged. The lungs were mottled with cranioventral consolidation. There was an approximately 2 cm diameter thrombus attached to the right atrioventricular valve. The small and large intestines were filled with fluid digesta and gas. The gallbladder contained a pasty white fluid. There were no gross lesions noted on examination of the brain or spinal cord.
Histopathologic Description:
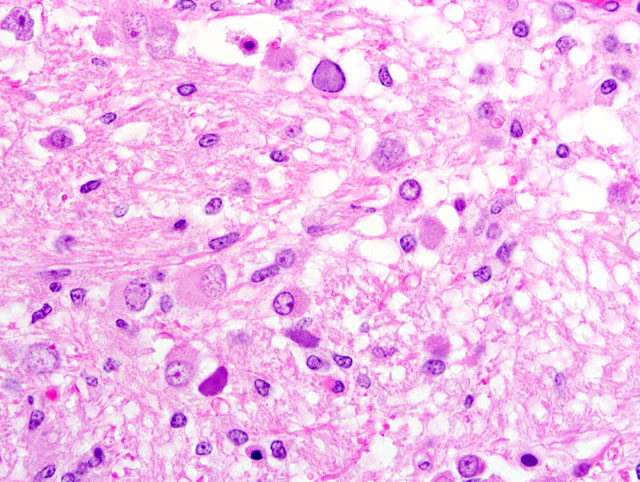
Brain (telencephalic/diencephalic junction- including thalamus, lateral ventricle, and substantia nigra; some sections are composed of mesencephalon): Replacing extensive regions of the white matter and multifocally spreading into the adjacent gray matter are variably sized foci of liquefactive necrosis. These foci contain large numbers of macrophages that contain abundant, intracytoplasmic, degenerate myelin (gitter cells) and are admixed with fewer lymphocytes and reactive astrocytes (some of which are multinucleate). The adjacent neuroparenchyma is loosened (edema) and contains large numbers of reactive astrocytes (gemistocytes) and rod-shaped microglia (
Fig 1). Both within the foci of liquefactive necrosis and in the adjacent neuroparenchyma are large numbers of astrocytes that are characterized by large, swollen nuclei that contain a single, round, 10-20μm, amphophilic, glassy inclusion body (
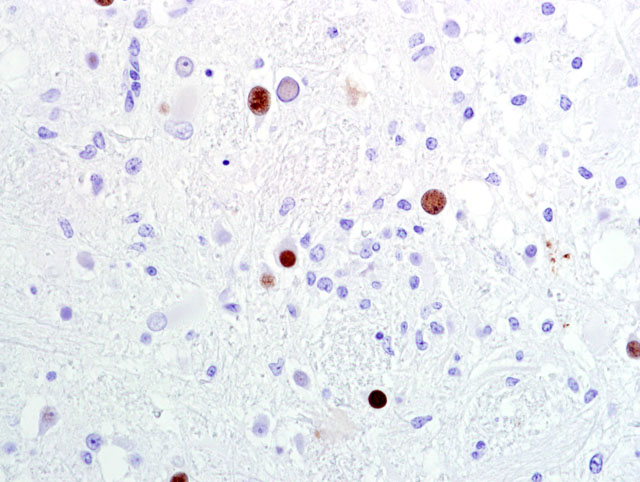
Fig 2). There are multiple foci of gliosis in the adjacent neuropil and occasional perivascular cuffs of lymphocytes are present within the gray matter. A small section of lateral ventricle is present in this section and the ependyma lining the lateral ventricle is irregular, proliferative, and broken in multiple sites. The ependymal cells have abundant cytoplasm and large, reactive nuclei and there are small numbers of lymphocytes within the ependyma. Blood vessels throughout the lesions are reactive and branched and typically are lined by reactive, hypertrophied endothelium. Immunohistochemistry for SV40 confirmed CNS infection.
Morphologic Diagnosis:
Brain (thalamus/substantia nigra): Severe, multifocal, chronic necrotizing encephalitis with intranuclear polyomaviral inclusions, marked astrocytosis and gliosis.
Lab Results:
CBC and blood chemistry were within normal limits.Â
Condition:
Polyomavirus infection
Contributor Comment:
Simian virus 40 (SV40) is a non-enveloped, oncogenic, double-stranded, DNA virus belonging to the Papovaviridae family and the Polyomavirinae subfamily. The viral genome consists of early- and late-coding regions and a non-coding regulatory region. The early region encodes DNA binding proteins, known as large or small T (tumor) antigens, which bind to the regulatory region of the genome to facilitate replication of late region viral DNA, which encodes three capsid proteins (VP1, VP2, VP3) that display genus-specific antigenic determinants. Polyomaviruses cause tumors when inoculated into species (e.g. hamsters) unrelated to their natural host.(1,2) Other primate polyomaviruses are: JC virus and BK virus, which infect humans; SA12 isolated from a South African vervet monkey kidney culture; B-lymphotrophic polyomavirus (LPV) isolated from an African green monkey lymphoblast cell line; baboon polyomavirus isolated from baboon kidney cell culture; and cynomolgus polyomavirus (CPV) isolated from immunosuppressed cynomolgus monkeys. Other non-primate mammalian polyomaviruses include Murine polyomavirus (MPyV), Hamster polyomavirus (HaPyV), Bovine polyomavirus (BPyV), Rabbit kidney vacuolating virus (RKV), Murine pneumotropic virus, and finally various avian polyomaviruses including budgerigar fledgling disease virus (BFDV).(2)
Polyomaviruses are virtually ubiquitous and harmless in healthy hosts, but can cause severe disease in immunocompromised individuals. In immunocompetent natural hosts, polyomaviruses remain latent within renal tissue and likely the central nervous system, with periodic asymptomatic reactivation of latent virus during pregnancy, diabetes, or old age. In immunocompromised SIV-infected macaques with simian AIDS, primary infection or reactivation of a latent infection results in interstitial nephritis, interstitial pneumonia, and either meningoencephalitis (ME) or progressive multifocal leukoencephalopathy (PML). In human AIDS patients, PML occurs in up to 5% of affected individuals and is considered an AIDS-defining lesion. It is characterized by multifocal demyelination primarily of the white matter due to infection of oligodendrocytes. The ME variant is diffuse and distinct from PML, affecting cerebral gray matter without demyelination.(1,3) PML is characterized by variably sized foci of necrosis that start in the white matter and merge into the adjacent gray matter. Reactive astrocytosis is a common finding around the lesions. Inclusion bodies are typically numerous and are most often found in astrocytes.Â
Cases of PML and ME are rare in the primate literature. The ME manifestation is typically seen in animals with concurrent nephritis, as tubular nephritis has been reported to be a feature of primary SV40 infection but not SV40 reactivation in SIV infected macaques. The current animal had mild interstitial nephritis; however, no SV40 antigen could be found by immunohistochemistry. In addition to polyomavirus, this Rhesus macaque had several other AIDS defining opportunistic infections, including biliary, pancreatic and tracheal cryptosporidiosis and adenoviral enteritis. Other AIDS defining lesions that were not present in this animal include
Pneumocystis sp. pneumonia and Cytomegalovirus infection.
A spontaneously occurring disease with characteristics similar to PML has been described in eight macaques. This observation was published in 1975, years before the discovery of SIV, and immunological competence was not determined in the monkeys reported.(4,5) Other virally-induced CNS lesions with inclusion bodies in non-human primates include CMV-associated meningoencephalitis; poliomyelitis virus-induced poliomyelitis; and measles virus-induced encephalitis. CMV-associated meningoencephalitis is differentiated from SV40-induced PML by the characteristic feature of cytomegaly with prominent intranuclear inclusion bodies. In addition, CMV associated encephalitis is not associated with necrosis and is typically more localized to the meninges. Poliomyelitis infection in non-human primates is rare and is restricted to the gray matter. Measles virus can affect the gray and white matter; however, the inclusion bodies are found both in the nucleus and the cytoplasm and are similar to other morbillivurses.(5,6) Finally, SIV-associated encephalitis has frequently been described in literature; however, the lesions in SIV encephalitis are present in both gray and white matter and composed of multifocal glial nodules, perivascular cuffs, and perivascular/meningeal aggregates of giant cells that lack inclusion bodies.(5,7)
References:
1. Horvath CJ, Simon MA, Bergsagel DJ, Pauley DR, King NW, Garcea RL, Ringler DJ: Simian virus 40-induced disease in rhesus monkeys with simian acquired immunodeficiency syndrome. Am J Pathol
140(6):1431-40, 1992
2. Imperiale MJ, Major EO: Polyomaviruses.Â
In: Fields Virology, eds. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, and Straus SE, 5th ed., vol. 2, pp. 2263-98. Lippincott Williams & Wilkins, Philadelphia, PA, 2007
3. Simon MA, Ilyinskii PO, Baskin GB, Knight HY, Pauley DR, Lackner AA: Association of simian virus 40 with a central nervous system lesion distinct from progressive multifocal leukoencephalopathy in macaques with AIDS. Am J Pathol
154(2):437-46, 1999
4. Gribble DH, Haden CC, Schwartz LW, Henrickson RV: Spontaneous progressive multifocal leukoencephalopathy (PML) in macaques. Nature
254(5501):602-4, 1975
5. Cusick PK, Morgan SJ: Nervous System.Â
In Nonhuman Primates in Biomedical Research: Diseases, eds. Bennett BT, Abee CR, Hedrickson R, 1st ed., pp. 461-83. Academic Press, 1998
6. Steele MD, Giddens WE Jr, Valerio M, Sumi SM, Stetzer ER: Spontaneous paramyxoviral encephalitis in nonhuman primates (
Macaca mulatta and
M. nemestrina). Vet Pathol
19(2):132-9, 1982
7. Sharer LR, Baskin GB, Cho ES, Murphey-Corb M, Blumberg BM, Epstein LG: Comparison of simian immunodeficiency virus and human immunodeficiency virus encephalitides in the immature host. Ann Neurol
23 Suppl:S108-12, 1988