Signalment:
2-year-old, female wild boar (
Sus scrofa).An adult wild boar was caught during boar hunting.
Gross Description:
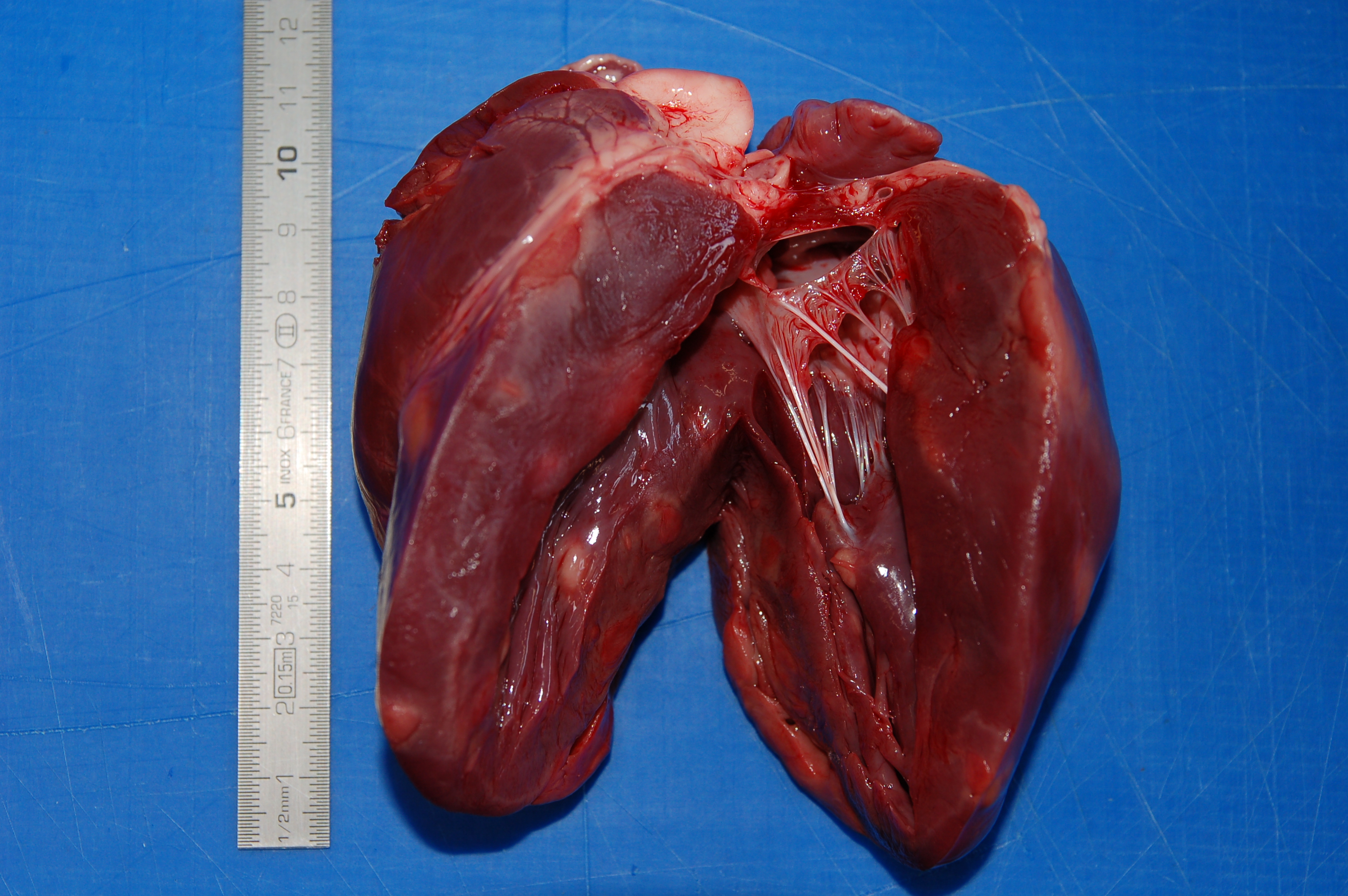
On post mortem examination, the boar was in good body condition with adequate muscle mass
and body fat stores. Within the ventricular walls and the interventricular septum there were numerous, multifocal to
coalescent, well demarcated, unencapsulated, white to pale pink, from 3 mm to 2 cm, irregularly round to oval
nodules that expanded and replaced the myocardial tissue and that elevated the epicardium and the endocardium,
protruding within the ventricular cavities. On cut surface the nodules were homogeneous in color and diffusely
firm.
Histopathologic Description:
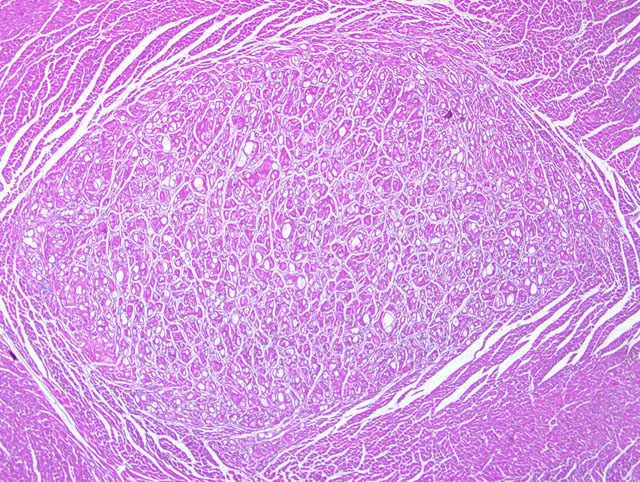
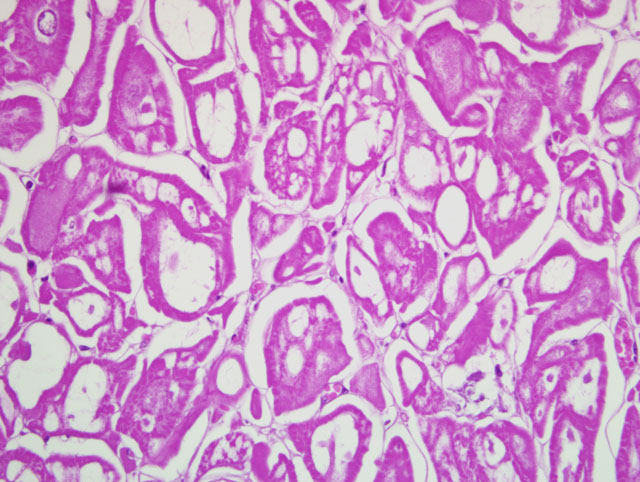
Heart: Within the ventricular wall there are multiple, focally extensive, welldemarcated,
not infiltrating, irregularly round to ovalar, intramuscular and subepicardial, from 3 mm to 1 cm in size
nodules that expand and replace the normal myocytes, composed of haphazardly disposed, irregularly shaped
myocytes with markedly distended cytoplasm characterized by single or multiple, clear, up to 200 um diameter
vacuoles occasionally containing a moderate amount of finely granular, eosinophilic material. The small amount of
remaining cytoplasm and the compressed nucleus are displaced to the periphery of the cells. There are numerous
cells characterized by a centrally located, up to 40 um in size, irregularly round nucleus, with one or two
indentations, marginated chromatin and a prominent nucleolus. These cells have abundant finely granular
eosinophilic cytoplasm. Minimal, diastase-labile, PAS-positive granules confirmed the presence of intracytoplasmic
glycogen within many of the cells. The anisocytosis and anisokaryosis are moderate. The mitotic index is less than
one. Multifocally within the nodules there are minimal inflammatory infiltrates composed of lymphocytes,
macrophages and plasma cells. There is mild diffuse edema and hyperemia. At the periphery of the nodules there
are multifocal hypereosinophilic myocytes with fragmented cytoplasm and pyknotic or absent nuclei (necrosis).
Morphologic Diagnosis:
Heart, myofibers: severe, multifocal myofiber vacuolar degeneration
consistent with glycogen deposits, and myocyte dysplasia compatible with multiple cardiac rhabdomyoma
(rhabdomyomatosis), wild boar, suid.
Condition:
Rhabdomyomatosis
Contributor Comment:
Cardiac rhabdomyoma is a lesion characterized by large vacuolated myocardial cells
containing glycogen, and synonymously rhabdomyomatosis, congenital glycogen tumor, circumscribed
glycogenic storage disease, nodular glycogenic degeneration, nodular glycogenosis and nodular glycogenic
infiltration are used for the lesion.(2) This kind of lesion typically occurs in children less than one year old, in pigs,
and rarely in cattle, sheep, dogs and deer.(3)
In swine it is an incidental finding and occurs most commonly in the left ventricle of the heart. Some authors
suggest a familial predisposition for cardiac rhabdomyomas in red wattle and red wattle-cross piglets.(4) Animals of
all ages are affected and the occurrence of this lesion in animals as young as three weeks old suggests a congenital
condition. The etiology and pathogenesis are not known and it is thought to be a hamartoma or malformation rather
than a true neoplasm. Ultrastructural and immunohistochemical studies in swine suggest it is a congenital dysplasia
of cardiac muscle and/or Purkinje cells. In fact, cardiac rhabdomyoma cells share ultrastructural features of both
cardiac myofibers and Purkinje cells, creating uncertainty as to their histogenesis.(4) The relative paucity of poorly
oriented myofibrils, abundant glycogen, binucleation, and desmosomal intercellular junctions in rhabdomyomas are
characteristics of Purkinje cells (4), but intercalated discs, which are exclusive to cardiac myofibers, are also present
in some rhabdomyoma cells.(4) This combination of ultrastructural features has led to the hypothesis that cardiac
rhabdomyomas arise from either two types of fibers or a pluripotential embryonic cell.(4)
Affected animals are usually asymptomatic but in affected dogs chylopericardium and right-sided congestive heart
failure have been reported.(2) The occurrence of cardiac rhabdomyomas in stillborn and neonatal red wattle piglets
in the absence of heart failure concurs with previous reports of the congenital and incidental nature of these lesions
in pigs.(4) However, the absence of concurrent disease in one red wattle piglet suggests that cardiac rhabdomyomas
may potentially cause sudden death, perhaps by interfering with normal myocardial conduction, as occurs in people.
(4) Grossly the lesions appear as irregular, pale, white, pink or tan myocardial foci or streaks varying in diameter
from barely visible to 3 cm in swine.
JPC Diagnosis:
Heart, myocardium: Cardiomyocyte swelling and glycogen-type vacuolar change, nodular,
multifocal, moderate to marked (rhabdomyomatosis).
Conference Comment:
In humans, cardiac rhabdomyomas occur most often in pediatric patients with tuberous
sclerosis, a hereditary autosomal dominant syndrome that results from mutations in the gene
TSC1 or more
commonly,
TSC2, characterized by the development of a variety of hamartomas and benign neoplasms. The gene
products, hamartin and tuberin, combine to form an inhibitor of the kinase mTOR, an important regulator of protein
synthesis, anabolic metabolism, and cell size. Interestingly, the tumors associated with tuberous sclerosis (e.g. giantcell
astrocytomas and cardiac rhabdomyomas) are noted for having voluminous cytoplasm.(1)
During tissue processing, the loss of glycogen from affected cells creates a distinctive and helpful histologic artifact:
spider cells, so named because of the radial arrangement of residual sarcoplasm that extends from the nucleus.(5)
Conference participants briefly discussed glycogen storage diseases in the differential diagnosis for this lesion.
In addition to the histopathologic findings described by the contributor, some sections reviewed by conference
participants contained few sarcocysts.
References:
1. Frosch MP, Anthony DC, De Girolami U: The central nervous system.Â
In: Robbins and Cotran Pathologic Basis
of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., pp. 1342-1343. Saunders Elsevier,
Philadelphia, PA, 2010
2. Kizawa K, Furubo S, Sanzen T, Kawamura Y, Narama I: Cardiac rhabdomyoma in a beagle dog. J Toxicol
Pathol
15:69-72, 2002
3. Kolly C, Bidaut A, Robert N: Cardiac rhabdomyoma in a juvenile fallow deer (Dama dama). J Wildl Dis
40:603-606, 2004
4. McEwen BJE: Congenital cardiac rhabdomyomas in red wattle pigs. Can Vet J
35:48-49, 1994
5. Radi ZA, Metz A: Canine cardiac rhabdomyoma. J Toxicol Pathol
37:348-350, 2009