Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 15
January 17th, 2018
CASE II: E 6940/16 (JPC 4100856).
Signalment: 4-week-old, female, Chilean flamingo, Phoenicopterus chilensis, avian.
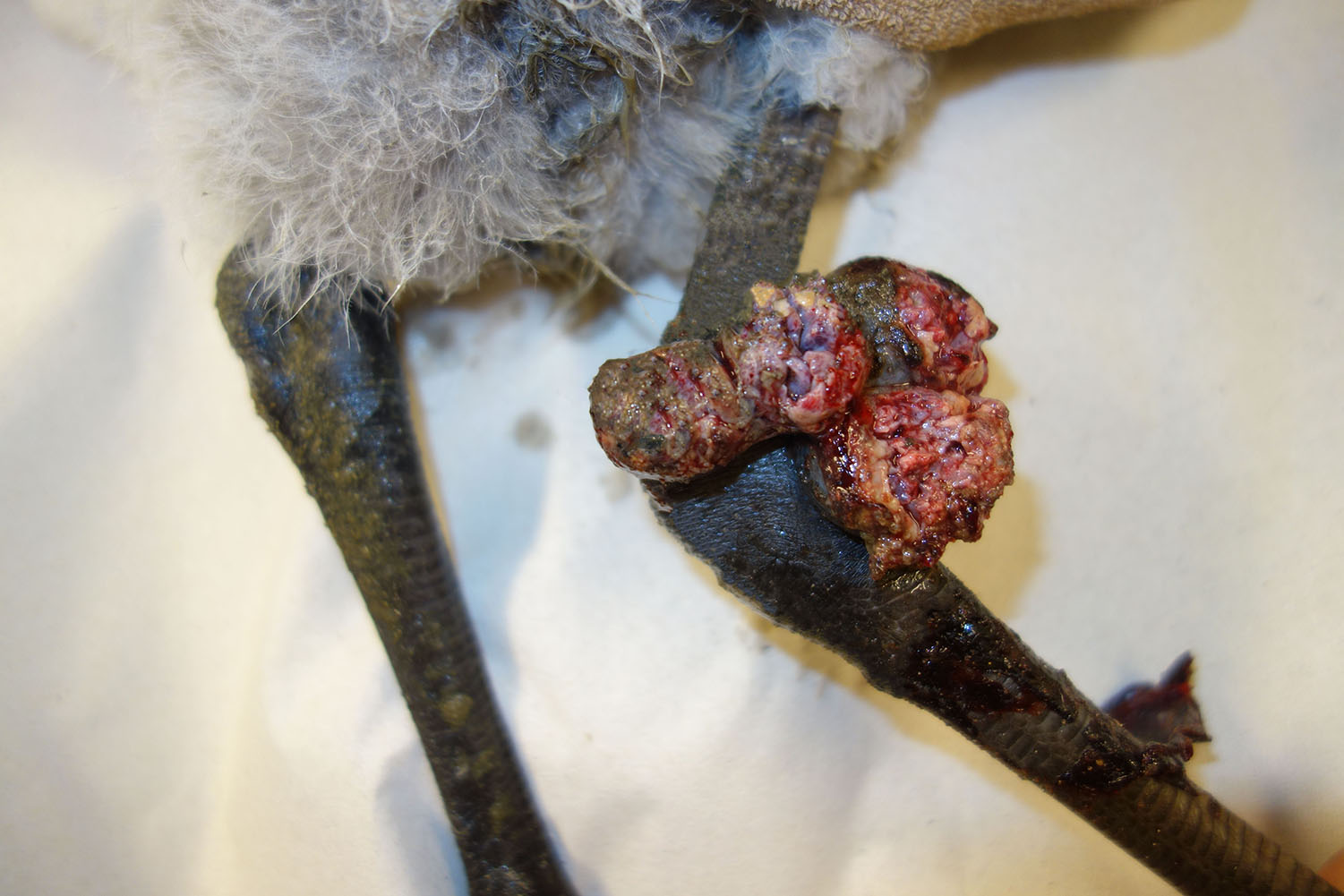
History: A 4-week-old, female flamingo from the zoo showed a multinodular, ulcerated, wart-like proliferation of approximately 5 x 4 x 3 cm extension in the skin at the right tibiotarsal joint. The proliferated tissue was surgically resected, fixed in 10% neutral buffered formalin and submitted for histological examination.
Gross Pathology: The submitted tissue was partially ulcerated and had a tan color. On cut surface it appeared multilobulated.
Laboratory results:
Formalin-fixed paraffin-embedded tissue (FFPE) was used for molecular sequencing of the gene encoding the 4b-protein. Phylogenetic analysis revealed a sequence homology of 99.9% with the ATCC strain of canarypox (genus: avipoxvirus).
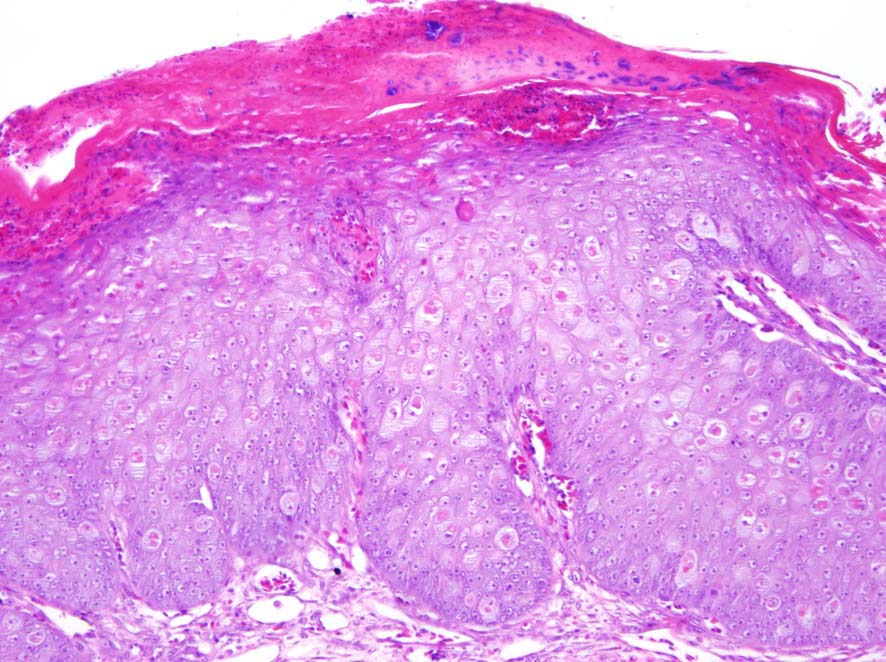
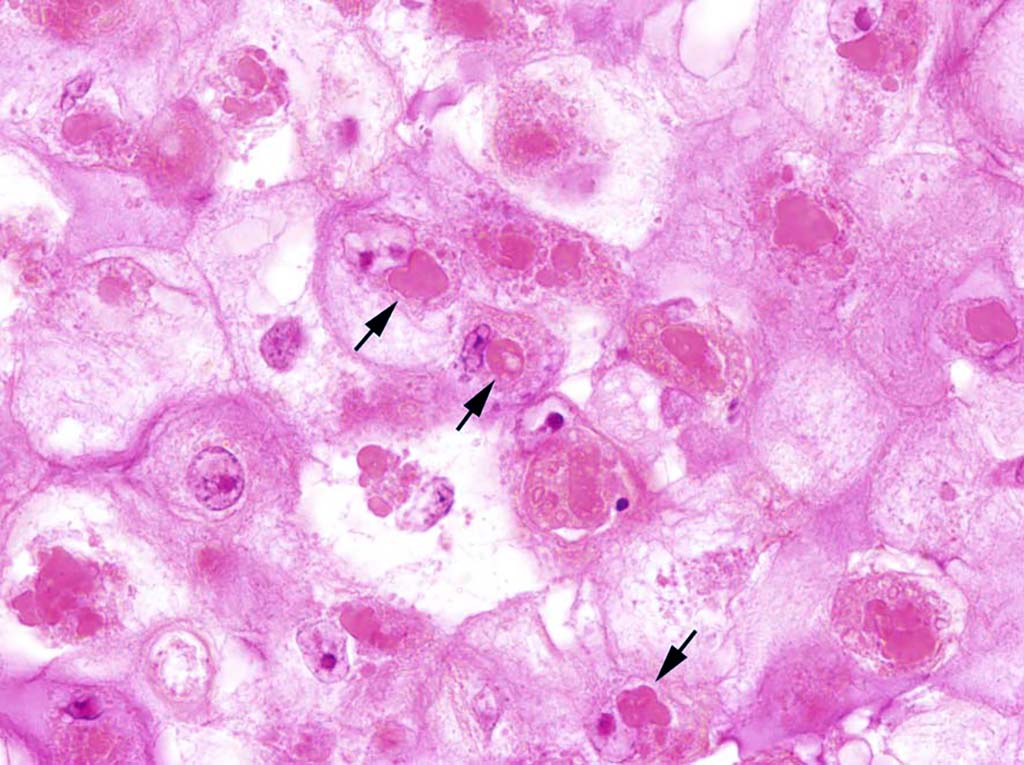
Microscopic Description: Glaborous skin: The epidermis of the featherless skin is irregularly proliferated and severely thickened with increased layers of spinosum cells (acanthosis). There are multifocal superficial or complete losses of the epidermis associated with extravascular erythrocytes and few heterophilic granulocytes. Underneath the stratum corneum there are multifocal accumulations of partly degenerated heterophilic granulocytes, erythrocytes, proteinaceous fluid and bacteria. Particularly, cells of the spinosum layer display severe diffuse hypertrophy with intracellular edema (hydropic degeneration). Eosinophilic inclusion bodies up to 15 µm in diameter are present in the cytoplasm (Bollinger’s inclusion bodies). Within the dermis, there is a diffuse mild to moderate infiltration of heterophilic granulocytes and few macrophages. Furthermore, numerous blood vessels are markedly extended and filled with red blood cells. Multifocally there are moderate accumulations of extravascular erythrocytes (hemorrhages) and eosinophilic, fibrillary material (fibrin).
Contributor’s Morphologic Diagnosis: Skin: Dermatitis, erosive and ulcerative, heterophilic, acute, diffuse, severe with epidermal hyperplasia, pustules, hydropic degeneration of keratinocytes and cytoplasmic, eosinophilic inclusion bodies (Bollinger’s inclusion bodies) consistent with poxvirus infection.
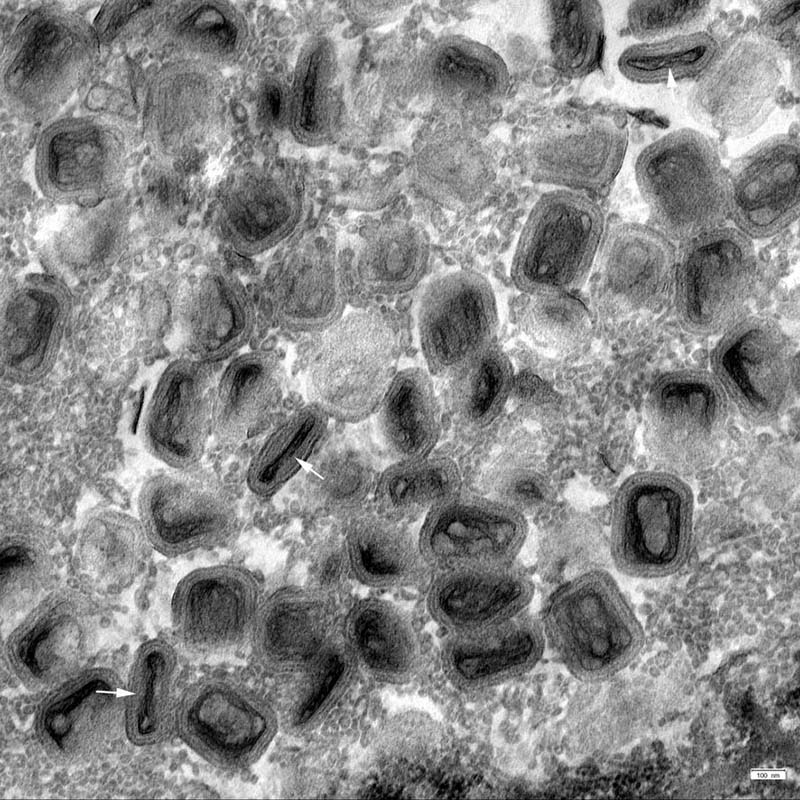
Contributor’s Comment: The morphological findings are consistent with a poxvirus infection that was confirmed by transmission electron microscopy. Molecular analysis revealed a canarypox strain of the genus avipoxvirus (APV). The histologic key lesions include epidermal hyperplasia and hydropic degeneration of keratinocytes with large, cytoplasmic, eosinophilic inclusion bodies (Bollinger’s inclusion bodies). Using the pop-off technique,11 transmission electron microscopy (TEM) revealed biconcave brick-shaped virions measuring 250 x 320 nm. Virus particles exhibited, depending of the sectioning plane, a biconcave core, two lateral bodies and an envelope consistent with avipox virions.15
Macroscopically, an exophytic ulcerated multinodular proliferation was present on the featherless skin at the tibiotarsal joint. This wart-like lesion represents the proliferative or cutaneous form of an APV infection (“dry pox”).8 It is characterized by nodular proliferations on featherless skin such as legs, feet, eyelids and base of the beak. Scars may be visible after recovery and healing. Another manifestation of APV infections is termed diphtheritic/diphtheroid or “wet” form that is characterized by proliferative and fibrino-necrotic lesions of the mucous membranes, predominantly of the tongue, pharynx and larynx.4,8,20 Birds may also show both forms. The mortality rate of the diphtheritic form is reported to be higher compared to the cutaneous form. However, secondary bacterial infections may significantly increase the mortality rate in the cutaneous form.20 Rarely, a septicemic form develops that is characterized by acute onset of ruffled plumage, somnolence, cyanosis and anorexia. This form may cause mortality rates of up to 99% and is seen predominantly in canaries and canary-finch crosses.8
Avian poxviruses belong to the genus Avipoxvirus which is a member of the subfamily Chordopoxvirinae with the family of Poxviridae. Avian poxviruses are large, brick-shaped, enveloped viruses with a double-stranded DNA. In infected cells, they replicate in the cytoplasm.10,19 Transmission occurs through latently infected birds and biting arthropods. In addition, direct transmission of the virus may be facilitated by small traumatic injuries caused by territorial behavior. Furthermore, virus may be transmitted through aerosols via mucous membranes of the eyes or the upper respiratory and digestive tracts.4,8,10,12 Mosquitos may retain infectious virus in the salivary glands for 2 to 8 weeks.8 Dry scabs can harbor the virus for many months.15 Birds of all ages are susceptible, however, mostly young individuals are affected. The incubation period varies from 7 to 14 days.14 The virus is usually named by the species in which it was originally isolated. Most investigations about mortality and morbidity of APV infections are based on single APV isolates, which make it difficult to find general information on pathogenicity of particular APV isolates in different species. For example, canaries are highly susceptible to canary poxviruses but they are resistant to pigeon pox, turkey pox and fowl pox.19 Nevertheless, APV can also cross species barriers and may infect taxonomically different species.10 Avian poxvirus has a worldwide distribution and infection is described in over 232 avian species in 23 orders.2 Disease can arise in domestic, pet and wild birds of many different species.19
Avipoxvirus infections have been described in various flamingo species in different countries of the world.10,13,16,20 American flamingos (Phoeniconais ruber) were infected in the USA and Portugal,10,13 Lesser flamingos (Phoenicopterus minor) in South Africa,16 and Greater flamingos (Phoenicopterus roseus) in Japan.16 In the presented case, a Chilean flamingo (Phoenicopterus chilensis) was affected. In all published cases, infection occurred in young individuals up to 4.5 months of age. They all suffered from the cutaneous form of avian poxvirus infection.8,11,16,20 In Portugal, Japan and the USA, single animals were affected,4,10,13 whereas in South Africa 30% of the fledgling flamingos displayed the cutaneous form.20
Identification and differentiation of various Avipoxvirus species is mainly based on sequencing of the 4b core polypeptide. This gene is composed of 1971 nucleotides and encodes a protein that has a molecular weight of 75.2 kDa.1,18 For the isolation of Avipoxvirus, the chorioallantoic membrane (CAM) of specific-pathogen-free (SPF) chicken embryos is inoculated. Within this culture system the virus forms type A cytoplasmic inclusions.2,10,13
As morphological differential diagnoses neoplastic proliferations, granulomatous inflammation as well as exuberant granulation tissue have to be considered.
JPC Diagnosis: Skin: Dermatitis, necrotizing and proliferative, focally extensive, severe, with ballooning degeneration, and intracytoplasmic eosinophilic viral inclusion bodies (Bollinger bodies), Chilean flamingo (Phoenicopterus chilensis), avian.
Conference Comment: The term fowlpox was initially used to describe poxvirus infections of all birds, but as the number of species affected grew, it became used specifically for the disease in chickens.3 Avianpox is an old disease that was previously thought to be related to human small pox and chicken pox. While this disease does not affect the human population, it does affect numerous avian species that we know of (chickens, turkeys, pigeons, canaries, psittacines, and wild birds) but perhaps all bird species are susceptible.17
The first USDA license issued for a poultry product was for the fowlpox vaccine in 1918.5 To this day, the pox vaccine is the primary method of disease control and prevention with initial vaccination of birds at 4 weeks of age or at any age if necessary. The fowl pox vaccine is currently being used as a vector for recombinant vaccines due to its efficacy and prevalence.17 The characteristic inclusion bodies of poxviruses (described above) represent the site of DNA synthesis and packing of the infectious virus particles. Avianpox viruses contain numerous genes for DNA replication, repair, and processing, as well as a specific enzyme (CPD photolyase) that repairs UV-induced DNA damage using visible light as a source of energy. This may help explain the virus’ environmental durability. Poxviruses encode proteins that affect host cells such as vaccinia virus growth factor (VGF) which stimulates proliferation of keratinocytes using epidermal growth factor receptors (EGFRs). Still other proteins inhibit complement mediated cell lysis and the host inflammatory response. All of these factors function to not only provide the virus a safe environment to replicate in, but also provide fertile soil for secondary bacterial infections.3
Gross differentials for cutaneous (dry) pox include mite infections and bacterial pododermatitis. Cnemidokoptes mutans (“scaley leg mite”) lives primarily in unfeathered skin and causes thick, hyperkeratotic shanks with white, scaly crusts, and Cnemidokoptes gallinae (“depluming mites”) lives in basal feather shafts and causes breakage or complete loss of feathers and intense irritation.6 Finally, bacterial pododermatitis (“bumblefoot”) most commonly caused by Staphylococcus aureus results in purulent abscesses on the plantar surface of the foot due to penetrating wounds.7
Conference participants noted variable serocellular crust formation in some sections with prominent colonies of superficial bacteria admixed with hemorrhage.
Table 1: Select genera of the family Poxviridae.9,12
|
Genus |
Virus/Disease |
Major Hosts |
|
Orthopoxvirus |
Vaccinia virus |
Numerous: cattle, buffalo, swine, rabbits |
|
Cowpox* |
Rodents (reservoir), cattle, cats, elephants, rhinos |
|
|
Camelpox |
Camels |
|
|
Ectromelia (Mousepox) |
Mice, voles |
|
|
Monkeypox* |
NHPs, squirrels, anteaters |
|
|
Capripoxvirus |
Goatpox |
Goats, sheep |
|
Sheeppox |
Sheep, goats |
|
|
Lumpy skin disease virus |
Cattle, cape buffalo |
|
|
Suispoxvirus |
Swinepox virus |
Swine (vector= Hematopinus suis) |
|
Leporipoxvirus |
Myxoma virus |
Rabbits (Oryctolagus & Sylvilagus spp.) |
|
Rabbit fibroma virus, Hare fibroma virus |
Rabbits |
|
|
Squirrel fibroma virus |
Grey and red squirrels |
|
|
Avipoxvirus |
Fowlpox, canarypox, quailpox, etc |
Chickens, turkeys, peacocks, etc. |
|
Parapoxvirus |
Caprine parapoxvirus (Orf; contagious ecthyma)* |
Sheep, goats |
|
Bovine parapox (bovine papular stomatitis virus)* |
Cattle |
|
|
Pseudocowpox* |
Cattle |
|
|
Sealpox* |
Seals |
|
|
Parapoxvirus of red deer |
Red deer |
|
|
Molluscipoxvirus |
Molluscum contagiosum virus* |
NHPs, birds, dogs, kangaroos, equids |
|
Yatapoxvirus |
Yabapox virus & tanapoxvirus* |
NHPs |
|
Unclassified |
Squirrel poxvirus, fish (carp edema), horsepox |
*zoonotic
Contributing Institution:
http://www.tiho-hannover.de/kliniken-institute/institute/institut-fuer-pathologie
References:
- Binns MM, Boursnell ME, Tomley FM, Campbell J. Analysis of the fowlpoxvirus gene encoding the 4b core polypeptide and demonstration that it possesses efficient promoter sequences. 1989;170:288–291.
- Bolte AL, Meurer J, Kaleta EF. Avian host spectrum of avipoxviruses. Avian Pathol. 1999;28:415–432.
- Boulianne M. Viral diseases. In: Avian Disease Manual. 7th ed. Jacksonville, FL: American Association of Avian Pathologists, Inc.; 2013: 46-49.
- El-Abasy MA, El-Khyate FF, Adayel SA, Hefny HY, El-Gohary AEA. Ostrich pox virus infection in farms at some Northern Egyptian governorates. Alexandria J Vet Sci. 2016;49:80–89.
- Espeseth DA, Lasher H. Early history of regulatory requirements for poultry biologics in the United States. Avian Dis. 2010;54(4):1136-1143.
- Fitz-Coy SH. Parasitic diseases. In: Avian Disease Manual. 7th ed. Jacksonville, FL: American Association of Avian Pathologists, Inc.; 2013: 154-155.
- Fulton RM. Bacterial diseases. In: Avian Disease Manual. 7th ed. Jacksonville, FL: American Association of Avian Pathologists, Inc.; 2013: 134-135.
- Gerlach H. Viruses. In: Ritchie BW, Harrison GJ, Harrison LR, eds. Avian Medicine: Principles and Application. Lake Worth, FL: Wingers Publishing; 1994:862–948.
- Hargis AM, Myers S. The integument. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th St. Louis, MO: Elsevier; 2017:1039-1040.
- Henriques AM, Fevereiro M. Avian poxvirus infection in a flamingo of the Lisbon Zoo. J Zoo Wildl Med. 2016;47:161–174.
- Lehmbecker A, Rittinghausen S, Rohn K, Baumgärtner W, Schaudien D. Nanoparticles and pop-off technique for electron microscopy a known technique for a new purpose. Toxicol Pathol. 2014;42:1041–1046.
- Mauldin EA, Peters-Kennedy J. Integumentary system. In: Maxie, MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 1. 6th ed. St. Louis, MO: Elsevier; 2016:616-625.
- Mondal SP, Lucio-Martínez B, Buckles EL. Molecular characterization of a poxvirus isolated from an American Flamingo (Phoeniconais ruber rubber). Avian Dis. 2008;52:520–525.
- Pledger A. Avian pox virus infection in a mourning dove. Can Vet J. 2005;46:1143–1145.
- Ritchie BW. Avian Viruses: Function and Control. Lake Worth, FL: Winger’s Publ.; 1995:285–311.
- Terasaki T, Kaneko M, Mase M. Avian poxvirus infection in flamingos (Phoenicopterus roseus) in a zoo in Japan. Avian Dis. 2010;54:955–957.
- Tripathy DN, Reed WM. In: Swayne DE, ed. Diseases of Poultry. 13th ed. Ames, IA: Wiley-Blackwell; 2013: 333-349.
- Weli SC, Traavik T, Tryland M, Coucheron DH, Nilssen O. Analysis and comparison of the 4b core protein gene of avipoxviruses from wild birds: evidence for interspecies spatial phylogenetic variation. Arch Virol. 2004;149:2035–2046.
- Weli SC, Tryland M. Avipoxviruses: infection biology and their use as vaccine vectors. Virol J. 2011;8:49.
- Zimmermann D, Anderson MD, Lane E, van Wilpe E, Carulei O, et al. Avian poxvirus epizootic in a breeding population of Lesser Flamingos (Phoenicopterus minor) at Kamfers Dam, Kimberley, South Africa. J Wildl Dis. 2011;47:989–993.