Signalment:
Gross Description:
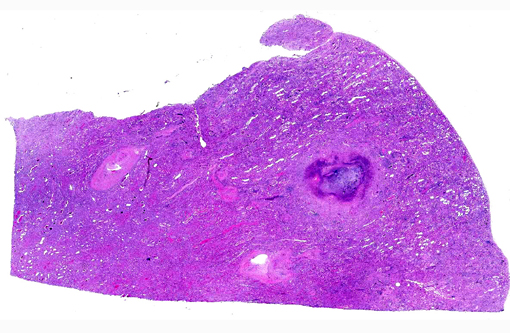
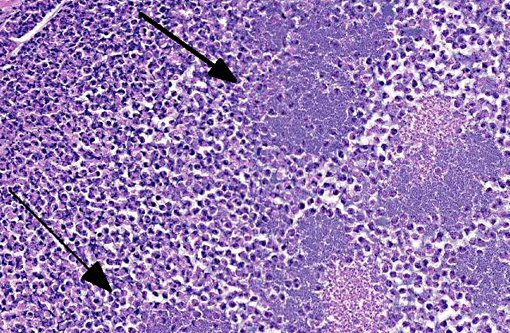
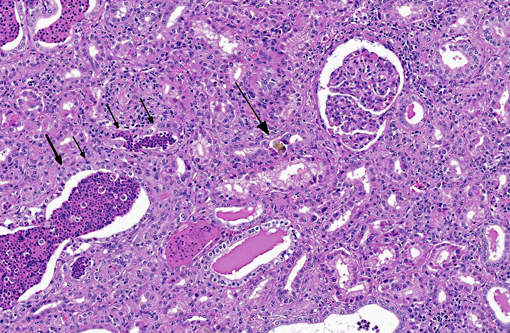
Histopathologic Description:
Special Stain: Gram-positive coccobacilli noted within necrotic foci.Â
Morphologic Diagnosis:
Kidney: Pyelonephritis, suppurative, diffuse, severe with tubular ectasia, proteinosis and scant coccobacilli.
Kidney: Nephrosis, multifocal, moderate with tubular necrosis, degeneration and regeneration, interstitial fibrosis, and numerous intratubular oxalate crystals.
Lab Results:
Sensitivity:
| Sulphafurazole | Sensitive |
| Trimethoprim | Resistant |
| Tetracycline | Sensitive |
| Erythromycin | Sensitive |
| Penicillin | Resistant |
| Novobiocin | Sensitive |
Hematology:
| Parameter | Value | Reference Range/ Units |
| RBC | 7.06 | 5.00-8.00 x 1012/L |
| PCV | 38 | 23-44 % |
| HGB | 14.6 | 8.0-15.0 g/dL |
| MCV | 54 | 44-62 fL |
| MCH | 21 H | 14-20 pg |
| MCHC | 38 H | 30-35 g/dL |
| WBC | 3.8 L | 4.0-12.0 x 109 /L |
| BANDS | 0.00 | 0.00-0.12 x 109 /L |
| NEUT | 0.95 | 0.60-4.00 x 109 /L |
| LYMPH | 2.58 | 2.50-7.50 x 109 /L |
| MONO | 0.27 | 0.03-0.84 x 109 /L |
| EOS | 0.00 | 0.00-2.40 x 109 /L |
| BASO | 0.00 | 0.00-0.20 x 109/L |
| NUCL.RBC | 3 | /100 WBC |
Biochemistry:
| BUN | 98.9 H | 2.1-10.7 mmol/L |
| CREA | 2550 H | 0-186 umol/L |
| BUN/CREA | 0.04 | 0.00-0.07 |
| PHOS | 3.45 H | 0.80-2.80 mmol/L |
| Ca | 2.60 | 2.00-2.75 mmol/L |
| TP | 105.9 H | 60.0-85.0 g/L |
| ALB | 36.0 | 25.0-38.0 g/L |
| Glob | 9.9 H | 30.0-45.0 g/L |
| Alb/Glob | 0.5 L | 0.7-1.1 |
| AST | 998 H | 0-120 U/L |
| GLDH | 19 | 0-30 U/L |
| GGT | 14 | 0-35 U/L |
| TBIL | 4.0 | 0.0-24.0 umol/L |
| CK | 3215 H | 0-300 U/L |
| MG | 1.05 | 0.74-1.44 mmol/L |
| BHB | 0.10 | 0.00-0.80 mmol/L |
| PROT-RTS | 130 H | 65-85 g/L |
| FIBRIN | 19 H | 3-7 g/L |
| PR/FI | 6 L | 15-100 |
Condition:
Contributor Comment:
The clinical pathology results support the gross and histological findings of severe renal disease, hepatic necrosis (histopathological finding, slide not included), and a significant inflammatory process (elevated immunoglobulins and fibrinogen). The leukogram shows a mild leukopenia, which in this case is likely to be due consumption of leukocytes as part of the inflammatory response. Rouleaux formation is a common finding in hyperglobulinemic or hyperfibrinogenemic states.(7)
Pyelonephritis is an inflammation of the renal pelvis and renal parenchyma, usually resulting from an ascending infection from the lower urinary tract.(3)
In cattle, Corynebacterium renal is a common cause of pyelonephritis.(2) In a survey of clinically affected animals, C. renale was the most common bacteria isolated(1) and in a slaughterhouse survey of cattle with gross kidney lesions, it was the third most predominant organism isolated.(6) It is a facultative commensal organism that is commonly isolated from the urinary tracts of healthy cattle.(1)
Other common causes of pyelonephritis in cattle include:(6,1)
- E.coli
- Truperella pyogenes (formerly Arcanobacter pyogenes)
- Corynebacterium cystitides
- Corynebacterium pilosum
- Streptococcus spp.
- Enterococcus faecalis
Acute pyelonephritis characteristically begins with necrosis and inflammation of the renal crest in an irregular pattern that, as the disease progresses, results in chronic changes where mononuclear cells replace neutrophils and fibrosis eventually predominates. Chronic pyelonephritis occurs more commonly in cattle than acute disease, with acute pyelonephritis often an incidental finding at post mortem.(3)
Vesicoureteral reflux is the most significant mechanism for transporting bacteria from the bladder to the kidney with refluxed urine occasionally being transported to the urinary space of the glomeruli.(3) Bacteria can spread hematogenously to the kidney, but this is a less common mode.Â
Within the kidney, the medullary region is the most susceptible to infection. This is due to its:
- Relative hypoxia (due to the low haematocrit in the vasa recta)
- Hypertonicity (depressed the phagocytic activity of leukocytes)
- High ammonia concentration (which interferes with the activation of complement).(3)
-
Several virulence factors make C. renale a significant pathogen for cattle. These include:
- Urea hydrolysis, which converts urea to ammonia. Ammonia initiates the inflammatory process and causes suppression of antibacterial defenses through complement inactivation.
- Pilli mediated attachment to the urothelium, which allows for persistence within the lower urinary tract in alkalotic urine. Cattle normally have alkalotic urine due to their herbivorous diet.(8) Oxalate nephrosis can also be a common finding in ruminants which may manifest as acute or chronic renal disease, abortion or hypocalcaemia without renal damage.(5)
- Fungi (in feedstuffs)
- Aspergillus niger
- Aspergillus flavis
- Some Penicillium spp.
- Ethylene glycol
- Primary hyperoxaluria- a rare genetic disorder that affects Beefmaster cattle
- Pyridoxine (vitamin B6) deficiency
- Excess ascorbic acid (vitamin C)reported in humans and a goat3
In ruminants, oxalate nephrosis is commonly caused by grazing plants high in soluble oxalate, typically those with greater than 2-2.5% soluble oxalate in dry matter. Many plants of this type contain greater than 10% soluble oxalate. Oxalate is higher in young plants with actively growing leaves. Soils high in nitrogen will also boost the concentration of oxalates.(4) It has been reported that annual deaths in sheep flocks grazing soursob (Oxalis pes-caprae) in Australia have had mortalities of 1%, although up to 25% of a flock may be affected.(5)
Naive or hungry cattle are more likely to suffer from oxalate toxicosis. Most oxalate containing plants are not readily eaten under normal grazing conditions and ruminal flora is not adapted to metabolize oxalates.(4)
Oxalate toxicosis occurs by calcium binding of the ingested soluble oxalates in the rumen, blood vessels liver and kidneys and leads to hypocalcaemia and oxalate crystal formation in the nephrons and blood vessels.(5)
The mechanism of injury in the kidney is from both mechanical obstruction of the nephrons and the cytotoxic effects of oxalate metabolites in the renal epithelium.(3)
Plants that are known to cause oxalate nephrosis in Australia and US include: Scientific Name Common Name Halogeton glomerulatus Halogeton, Barillia Chenopodium spp. Fat hen Sarcobatus vermiculatus Grease wood Rumex spp. Sorrel, dock Mesembryanthemum spp. Ice plants Tetragonia tetragonioides New Zealand spinach Trianthema spp. Black pig weed, Red spinach Amaranthoretroflexus Red root amaranth Beta vulgaris Beet Rheum x cultorum Rhubarb Oxalis pes-caprae* Soursob Portulaca oleracea * Pigweed Acetocella vulgaris Sheep Sorrel Atriplex muelleri Salt Bush Emex Australia Spiney emex Pennisetum ciliare Buffel grass Seratia sphacelata* Seratia Acetosa vesicara Ruby dock Brassica spp. *Denotes common causes of oxalate poisoning in Australia.(5)
Other substances that are known to cause oxalate nephrosis include:
Although commonly reported, hypocalcaemia associated with oxalate nephrosis is not often seen at out laboratory.
JPC Diagnosis:
1. Kidney: Pyelonephritis, suppurative and necrotizing, chronic, diffuse, severe with large colonies of bacilli.
2. Kidney, tubules: Oxalate crystals, multiple.
The contributor provides an excellent, thorough review of both pyelonephritis caused by C. renal infection and oxalate nephrosis. Microscopically, there are low to moderate numbers of intratubular calcium oxalate crystals with rare foci of minimal granulomatous response; however, these mild histopathologic features in combination with the serum biochemistry results (specifically the lack of hypocalcemia), led conference participants to conclude that oxalate toxicosis did not contribute significantly to the pathogenesis in this case. There was also some debate regarding the composition of the deeply basophilic material often present within necrotic renal tubules. Although most participants initially identified this substance as mineral, the moderator suggested instead that it is composed of aggregates of DNA secondary to widespread necrosis, pointing out its similarity to the microscopic appearance of material seen in acute tumor lysis syndrome, a condition seen in mice. As noted by the contributor, the clinical pathology findings are consistent with severe pyelonephritis due C. renale; however, the significant elevations in CK and AST could also result from hemolysis. Additionally, there is some slide variation; not all sections contain renal pelvis and in those that do, medullary tubules are often widely separated by fibrosis (demonstrated with Massons trichrome). A brief discussion ensued regarding the severity of the medullary fibrosis in this case, with the moderator suggesting that this was within normal limits for ruminants.
Conference Comment:
1. Braun U, Nuss K, Wehbrink D, et al. Clinical and ultrasonographic findings, diagnosis and treatment of pyelonephritis in 17 cows. The Veterinary Journal. 2008;175:240-248.
References:
2. Jones TC, Hunt RD, King NW. Veterinary Pathology. 6th ed. Baltimore, MD: Williams & Wilkins; 1997:1126-1131.
3. Maxie MG, Newman SJ. Urinary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol. 2. 5th ed. Philadelphia, PA: Elviser Saunders; 2007:470-494.
4. McKenzie RA. Australias Poisonous Plants, Fungi and Cyanobacteria: A Guide to Species of Medical and Veterinary Importance. Collingwood, Victoria: CSRIO Publishing; 2012:45-46.
5. McKenzie RA. Plant toxicology. In: Toxicology for Australian Veterinarians. 1st ed. Brisbane, Queensland; Ross A. McKenzie; 2002:28-36.
6. Rosenbaum A, Guard CL, Njaa BL, et al. Slaughterhouse survey of pyelonephritis in dairy cows. Vet Rec. 2005;157:652-655.
7. Stockham SL, Scott MA. Fundamentals of Veterinary Clinical Pathology. 2nd ed. Ames, IA: Blackwell Publishing LTD; 2008:393-396, 415-440, 677-681.
8. Takai S, Yanagawa R, Kitamura Y. pH-dependant adhesion of piliated Corynebacterium renale to bovine bladder epithelial cells. Infection and Immunity. 1980;28(3):669-674.