Signalment:
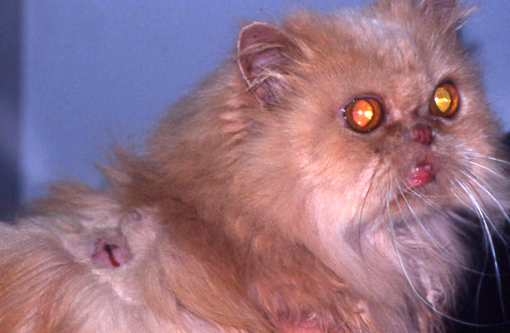
Gross Description:
Morphologic Diagnosis:
Lab Results:
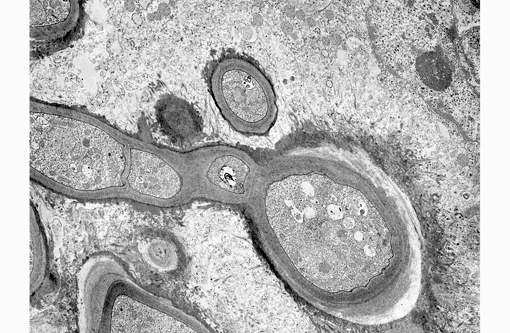
Electron Microscopy Description: Dermis, 12,000 magnification. On the electron micrograph, there are longitudinal and transverse sections of fungal hyphae within the cytoplasm of a reactive macrophage. On the right-hand top corner, two nuclei with slightly irregularly infolded nuclear envelopes, a prominent nucleolus, randomly scattered dispersed chromatin and multifocal aggregates of marginated chromatin are evident. The cytoplasm is abundant and contains mitochondria, stacks of rough endoplasmic reticulum and free ribosomes (binucleated macrophage).
The fungal hyphae are septate and characterized by a thick homogeneously moderately electrondense fibrillar cell wall with an underlying thin, highly electron-dense plasma membrane enclosing moderate amount of granular, moderately electron-dense cytoplasm. The latter contains few elongated to round mitochondria, moderate numbers of round single-membrane bound electron lucent vacuoles, scattered ribosomes and rare myelin figures. Two nuclei with central to paracentral prominent nucleoli and a nuclear envelope are present. Fungal structures are surrounded by a variably thick, moderately to highly electron-dense, fibrillar material (accumulation of immunoglobulins Splendore-Hoeppli reaction), multifocally radiating from the hyphal surface.
Condition:
Contributor Comment:
Specifically, electron microscopy of M. can is has been well described.(17) M. canis hyphae have a cell wall consisting of an outer thin, electron-dense layer and an inner and broader fibrillar electron-lucid layer. Septa are produced from the inner and broader fibrillar electron-lucid layer and are characterized by septal pores. The plasma membrane is a thin, electron-dense delimiting membrane contiguous with the inner surface of the cell wall. In the cytoplasm, a large central vacuole surrounded by an electron-dense tonoplast enclosing electron-lucent flocculent material is visible. Mitochondria having no polarity are scattered throughout the cytoplasm of the hyphae and can be filamentous or spherical. Mitochondria have double membrane and extensive cristae that might extend across the organelles. Hyphae have sparse narrow tubular endoplasmic reticulum and a large tonoplast. Glycogen is abundant and often clumped and lipid inclusions are also present. Single membrane bound vesicles with central bodies are also present at the margins of the hyphae. Hyphae are often bi- to multinucleated (as in the image provided). The nuclear membrane has a double envelope with intervening nuclear pores. Additional features are the presence of single membrane bound vesicles with central bodies with high electron opacity.
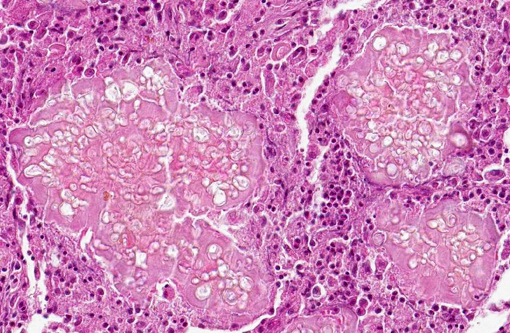
Clinical, gross and microscopic findings were representative of a deep dermatophyte infection consistent with feline dermatophytic pseudomycetoma. The disease associated with Microsporum canis has been described also in dogs,(1,8) horses(12) and humans.(2,7,10,14) Dermatophytic pseudomycetomas are uncommon to rare, deep cutaneous to subcutaneous fungal infections that produce tissue grains or granules. These tissue grains are composed of fungal aggregates embedded in amorphous eosinophilic material representing antigenantibody complexes (SplendoreHoeppli reaction).
Feline pseudomycetomas have been reported mostly in Persian cats.(3,4,5,8,9,11,15) Sporadic cases have been reported in Himalayan, Domestic Shorthair, and in Maine Coon cats.(8,11,13,16) Genetic predisposition has been hypothesized to play a role in the development of these lesions since the Persian breed seems predisposed also to conventional dermatophytosis.(8) Age and gender predilections have not been observed.
The frequent localization of the lesions in the dorsal trunk, most commonly in outdoor cats, suggests a traumatic implantation of organisms from hair follicles with dermatophytic colonization by biting or fighting.(8) Some authors hypothesize that mycelial elements reach the dermis from spontaneously ruptured hair follicles in association with dermatophyte infection. Once in the dermis, fungi aggregate and induce a granulomatous reaction. Positive dermatophyte cultures from normal-appearing areas distant from the dermatophytic pseudomycetoma indicate that affected cats may previously have been inapparent carriers. Inapparent carriage of Microsporum canis is common in Persian and Himalayan cats.(5,8)
Grossly, dermatophytic pseudomycetomas are characterized by one or more subcutaneous nodules that occur most commonly over the dorsal trunk or tail base.(5,8,9,11) Lesions are firm, irregularly shaped nodules that gradually enlarge and coalesce in the dermis or the underlying subcutaneous tissue. Lesions may fistulate and discharge a seropurulent to necrotic material. Systemic clinical signs are uncommon, but lymphadenomegaly may be present. Intra-abdominal dermatophytic granulomatous peritonitis sharing many features with pseudomycetoma has been reported in Persian cats.(3,15,18)
Although dermatophytic pseudomycetoma fulfils most of the criteria for true mycetomas (nodular inflammation with fibrosis, fistulae draining from deep tissue, presence of tissue grains), fewer hyphal elements are present, and the lesions apparently lack the cement substance that holds true mycetoma grains together.(8)
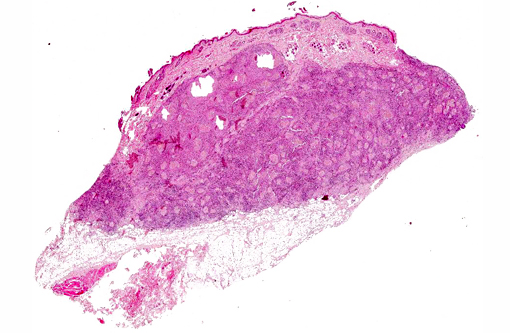
Microscopically, lesions are located mostly in the dermis where aggregates of grey, refractile and highly pleomorphic fungal hyphae are characteristically found. These are tangled and delicate, and contain numerous large, clear, bulbous, thick-walled dilatations, resembling spores. Smaller swellings within the hyphae create a vacuolated or bubbly appearance to these structures. The fungal aggregates are imbedded in amorphous eosinophilic material to form large tissue grains, or granules that are also visible grossly. SplendoreHoeppli reaction is brightly eosinophilic and locates around the periphery of organized aggregates of organisms. Granules are cuffed by and intermingled with large macrophages, giant cells, and variable, sometimes numerous neutrophils. Macrophages have abundant, granular cytoplasm. In some cases, fragments of hyphae are present within individual macrophages beyond the boundaries of tissue grains or granules. Reactive fibroblasts and collagen may surround or dissect the lesions often creating lobules composed of multiple granules and their attendant inflammation.(8,11)
Cytology has proven useful in the diagnosis of pseudomycetoma.(9,13,19) However, histopathology and fungal culture of biopsy specimens are required for a definitive and specific diagnosis. Organisms can be stained with periodic acid-Schiff, Gomori methenamine silver, Grocott stains and Fonata-Masson.(8,10) Microsporum canis has been isolated in typical cases of dermatophytic pseudomycetomas where fungal culture has been performed.(8) PCR from paraffin embedded tissues is also useful for M. canis identification and has been utilized in cases of feline, canine and equine lesions.(12) Immunohistochemistry using rabbit anti-Microsporum canis antiserum identified this agent also in two canine cases.(1)
Dermatophyte pseudomycetomas are considered difficult to manage clinically and the prognosis is considered poor in cats.(3,4,8) The lesions often recur after surgical excision alone(5) although in this case no recurrence was observed. There are contrasting reports regarding poor(4) or successful response of feline pseudomycetomas following terbinafine treatment.(13) Little response to griseofulvin, ketoconazole or itraconazole has also been reported.(5,13,18) However, a combination of surgical excision with adjunctive long term medical therapy has recently been reported to be successful.(5,18)
Clinical differential diagnoses should include cryptococcosis and other systemic mycoses, sporotrichosis, cutaneous infections of other opportunistic fungi, and neoplasia. The marked breed predilection for Persian cats is helpful in increasing the index of suspicion for dermatophytic pseudomycetoma. Histologically, most of the systemic and opportunistic fungi affecting cats and dogs are smaller and more uniform in appearance and do not form granules or grains in tissue.(8) Trichophyton mentagrophytes has been reported to cause dermatophytic granulomatous inflammation in cats but the lesions do not have the typical histologic features of dermatophytic pseudomycetoma, and are characterized by a more diffuse tissue reaction with associated heavy colonization of the keratin of hair follicles and epidermis.
JPC Diagnosis:
Conference Comment:
The contributor provides an eloquent discussion on this entity, highlighting the characteristic ultrastructural, histopathologic and gross findings while adeptly discussing clinical presentation, management and appropriate differentials worthy of consideration.
References:
1. Abramo F, Vercelli A, Mancianti F. Two cases of dermatophytic pseudomycetoma in the dog: an immunohistochemical study. Vet Dermatol. 2001;12:203-207.
2. Berg JC, Hamacher KL, Roberts GD. Pseudomycetoma caused by Microsporum canis in an immunosuppressed patient: a case report and review of the literature. J Cutan Pathol. 2007;34:431-434.
3. Black SS, Abernethy TE, Tyler JW, Thomas MW, Garma-Avi+â-¦a A, Jensen HE. Intra-abdominal dermatophytic pseudomycetoma in a Persian cat. J Vet Intern Med 2001;15:245-248.
4. Bond R, Pocknell AM, Tozet CE. Pseudomycetoma caused by Microsporum canis in a Persian cat: lack of response to oral terbinafine. J Small Anim Pract. 2001;42:557560.
5. Chang SC, Liao JW, Shyu CL, Hsu WL, Wong ML. Dermatophytic pseudomycetomas in four cats. Vet Dermatol. 2011;22:181-7.
6. Cheville NF. Ultrastructural pathology. Algae, fungi and other eukaryotes. In: An introduction to interpretation. 1st ed. Ames, IA: Iowa State University Press; 1994:761-787.
7. Colwell AS, Kwaan MR, Orgill DP. Dermatophytic pseudomycetoma of the scalp. Plast Reconstr Surg. 2004;113:1072-1073.
8. Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Infectious nodular and diffuse granulomatous and pyogranulomatous disease of the dermis. In: Skin Diseases of the Dog and Cat, Clinical and Histopathologic Diagnosis. 2nd ed. Oxford, UK: Blackwell Science Publishing; 2005:288-291.
9. Kano R, Edamura K, Yumikura H, Maruyama H, Asano K, Tanaka S, et al. Confirmed case of feline mycetoma due to Microsporum canis. Mycoses. 2009;52:80-83.
10. Kramer SC, Ryan M, Bourbeau P, Tyler WB, Elston DM. Fontana-positive grains in mycetoma caused by Microsporum canis. Pediatr Dermatol. 2006;23:473-475.
11. Miller RI. Nodular granulomatous fungal skin diseases of cats in the United Kingdom: a retrospective review. Vet Dermatol. 2010;21:130-5.
12. Nardoni S, Franceschi A, Mancianti F. Identification of Microsporum canis from dermatophytic pseudomycetoma in paraffin-embedded veterinary specimens using a common PCR protocol. Mycoses. 2007;50:215-217.
13. Nuttall TJ, German AJ, Holden SL, Hopkinson C, McEwan NA. Successful resolution of dermatophyte mycetoma following terbinafine treatment in two cats. Vet Dermatol. 2008;19:405-410.
14. Rinaldi MG, Lamazor EA, Roeser EH, Wegner CJ. Mycetoma or pseudomycetoma? A distinctive mycosis caused by dermatophytes. Mycopathologia. 1983;81:41-48.
15. Stanley SW, Fischetti AJ, Jensen HE. Imaging diagnosis sublumbar pseudomycetoma in a Persian cat. Vet Radiol Ultrasound. 2008;49:176-178.
16. Thian A, Woodgyer AJ, Holloway SA. Dysgonic strain of Microsporum canis pseudomycetoma in a domestic long-hair cat. Aust Vet J. 2008;86:324-328.
17. Werner HJ, Jolly HW Jr, Spurlock BO. Electron microscope observations on the fine structure of Microsporum canis. J Invest Dermatol. 1966;46:130-134.
18. Zafrany A, Ben-Oz J, Segev G, Milgram J, Zemer O, Jensen HE, et al. Successful treatment of an intra-pelvic fungal pseudomycetoma causing constipation and hypercalcaemia in a Persian cat. Feline Med Surg. 2014;16:369-72.
19. Zimmerman K, Feldman B, Robertson J, Herring ES, Manning T. Dermal mass aspirate from a Persian cat. Vet Clin Pathol. 2003;32:213-217.