Wednesday Slide Conference, Conference 13, Case 2
Signalment:
Adult, female, Sprague-Dawley rat (Rattus norvegicus)
History:
An adult, female, Sprague-Dawley rat, belonging to an intentional breeding colony was presented for diagnostic investigation of hind limb paresis. This animal had been fed Certified Rodent Diet 5002 (PMI Feeds, Inc) and given municipal water ad libitum, and only used for intravenous administration training without any active pharmaceutical ingredient. All procedures performed on the animal were in accordance with regulations and established guidelines reviewed and approved by an Institutional Animal Care and Use Committee.
Gross Pathology:
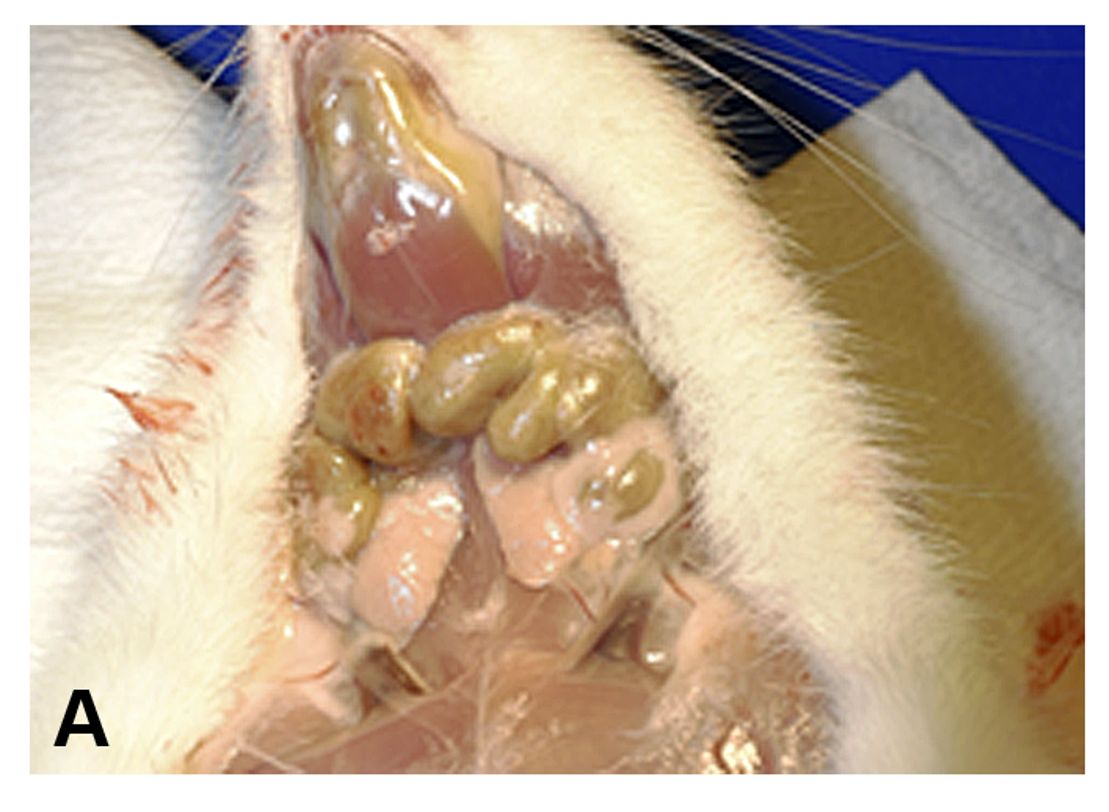
A gross examination revealed enlargement and greenish discoloration of multiple lymph nodes, including iliac, inguinal, mesenteric, popliteal, and mandibular lymph nodes. The spleen was also enlarged and the femoral bone marrow appeared green. A comprehensive set of tissues were collected in 10% neutral buffered formalin. All collected tissues were routinely processed and stained with hematoxylin and eosin (H&E) for light microscopic examination.
Microscopic Description:
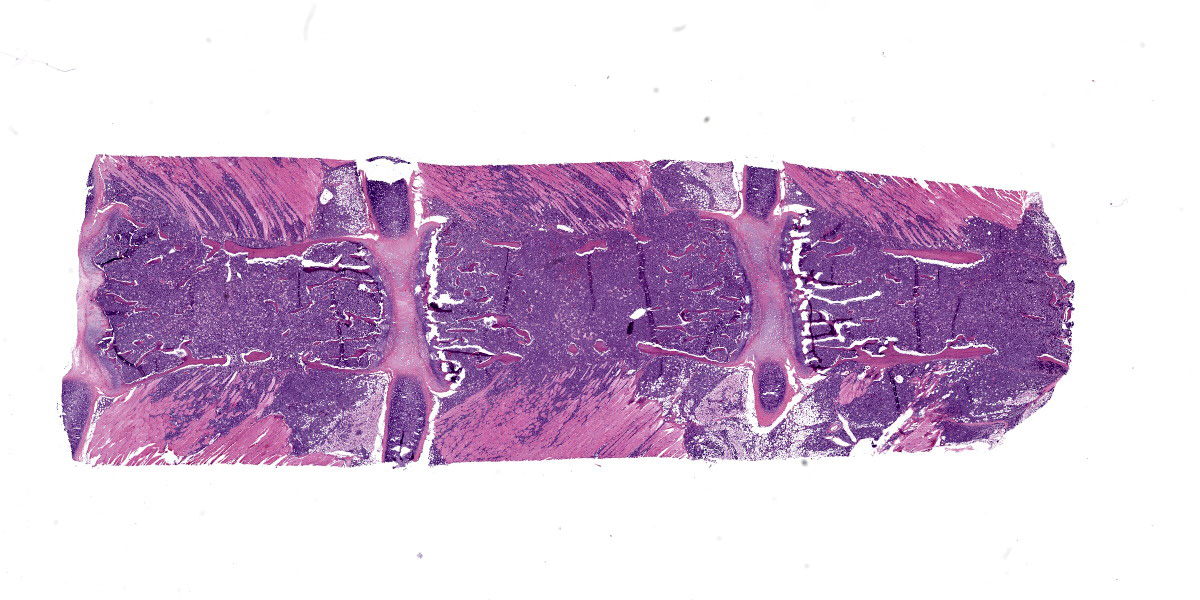
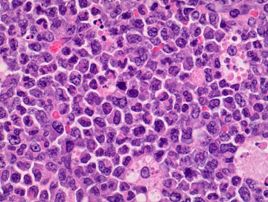
Sternum, bone marrow: Neoplastic cells had large, vesicular nuclei that varied in shape from round, segmented, lobulated, to ring shaped, and contained a small amount of slightly eosinophilic cytoplasm. They were noncohesive and arranged in diffuse sheets. There were approximately 5 mitotic figures per high power (400X magnification) field noted in the neoplastic cells in the bone marrow. Throughout the sheets of neoplastic cells in many of the affected tissues, there were small to moderate numbers of scattered large macrophages that contained phagocytized apoptotic cell debris.
Neoplastic cells completely replaced the normal hematopoietic elements in the medullary cavity of the sternum and had infiltrated into the surrounding bone, skeletal muscles, and joints.
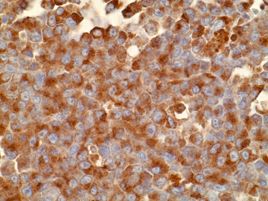
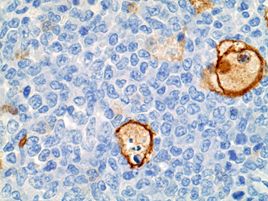
Representative sections were selected for Iba?1 and MPO (myeloperoxidase) immunohistochemistry (IHC). Immunohistochemically, neoplastic cells within the bone marrow had strong cytoplasmic immunoreactivity for MPO, which is the hallmark of the myeloid lineage. Cellular debris-laden macrophages scattered throughout the sheets of neoplastic cells had strong membranous immunoreactivity for Iba?1, a macrophage marker.
Contributor’s Morphologic Diagnosis:
Sternum/Bone marrow, myeloid leukemia in a Sprague-Dawley Rat
Contributor’s Comment:
The current case was diagnosed as myeloid leukemia based on the characteristic cytological morphology and immunohistochemistry profile of the neoplastic cells. Various amounts of nuclear indentation noted among neoplastic cells was indicative of granulocytic differentiation and was suggestive of a more chronic leukemic process. Diffuse immunoreactivity of neoplastic cells for MPO indicated that the cells stemmed from myeloid lineage because MPO is expressed by myeloid cells at all stages.
Massive inflammatory reactions to lesions in the skin or internal organs can stimulate compensatory myelopoiesis and result in remarkable extramedullary hematopoiesis and/or myeloid hyperplasia in the spleen and other organs as well as bone marrow, mimicking myeloid leukemia. The current case was differentiated from those compensatory reactions to inflammatory stimuli based on its invasive growth pattern, destruction of bone tissue associated with bone marrow proliferation, and the absence of inflammatory lesions.4,6
The macroscopic finding of green discoloration observed in the enlarged lymph nodes and bone marrow of the rat described in the current case report is associated with chloromas in humans, which has been described as a mass composed of immature granulocytic cells. This tumor occurs primarily in patients with myelogenous leukemia and typically has a green color caused by high levels of myeloperoxidase in those immature granulocytic cells.2
In the current case, the neoplasia was multicentric and with an invasive growth pattern. The presenting clinical finding of hind limb paresis was ascribed to extensive infiltration of neoplastic cells from the bone marrow through adjacent bones, joints, and skeletal muscles in the hind limb region. There was no evidence of tumor cell infiltration into the spinal cord or brain sections examined.
Hyaline droplets occurred in the renal proximal tubules in the current case. Hyaline droplets are known to accumulate in the identical segments of renal tubules in rats with a different neoplasm, histiocytic sarcoma. These droplets have been identified as lysozyme, which is a major secretory product of monocytes and macrophages.3 It is important to note that IBA1 immunohistochemistry results demonstrated that apoptotic figures scattered throughout the sheets of neoplastic cells were cellular debris-laden macrophages. As with lysozyme in the case of histiocytic sarcoma, excessive production of this protein by activated macrophages is considered to be the likely cause of renal accumulation.
Contributing Institution:
Pfizer Drug Safety Research and Development
455 Eastern Point Rd.,
Groton, CT 06340
https://www.pfizer.com/partners/research-and-development
JPC Diagnosis:
Bone marrow and skeletal muscle: Myeloid leukemia.
JPC Comment:
We agree with the contributor that the tissue submitted represents a hematopoietic neoplasm for the histologic features they so nicely capture in their discussion. Conference participants honed in on infiltration of the surrounding adipose tissue and muscle by neoplastic cells as well as the effacement of the marrow cavity and loss of trabecular and cortical bone as major features of this case.
We performed IHCs for IBA1, myeloperoxidase, lysozyme, CD3, CD33, CD34, CD56, CD117, PAX5, as well as an EBER ISH. We confirmed the background of tumor-associated macrophages with IBA1 (while also ruling out histiocytic sarcoma), though our results for MPO, lysozyme, CD33, and CD34 were equivocal and we could not reproduce the contributor’s IHC result. Curiously, we did note moderate immunoreactivity of neoplastic cells for CD3 and CD56 which suggests a potential (although we feel unlikely) potential T-lymphocyte origin for these cells, though our lab is not optimized for rodent IHCs. As such, we rendered a H&E diagnosis for the case. ISH and other IHCs for this case were unremarkable.
We previously covered hyaline droplets in rodents in a recent (WSC 24-25, Conference 6, Case 1) which featured a histiocytic sarcoma within the kidney itself. We also considered myeloid leukemia in that case – incidentally, the morphology of neoplastic cells is very similar. Ruleouts for hyaline droplets include histiocytic sarcoma,3 lymphoma,1 chronic progressive nephropathy, and alpha-2u globulin nephropathy.5
References:
1. Decker JH, Dochterman LW, Niquette AL, et al. Association of Renal Tubular Hyaline Droplets with Lymphoma in CD-1 Mice. Toxicol Pathol. 2012; 40: 651-655.
2. Ginsberg LE, Leeds NE. Neuroradiology of leukemia. AJR. 1995;165:525-534.
3. Hard GC, Snowden RT. Hyaline droplet accumulation in rodent kidney proximal tubules: An association with histiocytic sarcoma. Toxicol Pathol. 1991;19:88-97.
4. Long RE, Knutsen G, Robinson M. Myeloid hyperplasia in the SENCAR mouse: Differentiation from granulocytic leukemia. Environ Health Persp. 1986;68:117-123.
5. Swenberg JA, Short B, Borghoff S, Strasser J, Charbonneau M. The comparative pathobiology of alpha 2u-globulin nephropathy. Toxicol Appl Pharmacol. 1989 Jan;97(1):35-46.
6. Ward JM, Rehg JE, Morse HC. Differentiation of rodent immune and hematopoietic system reactive lesions from neoplasia. Toxicol Pathol. 2012;40:425-434.