Signalment:
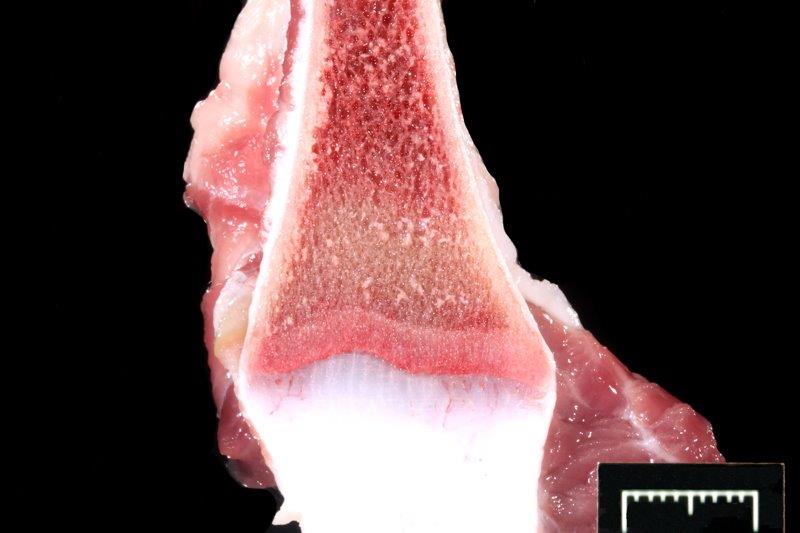
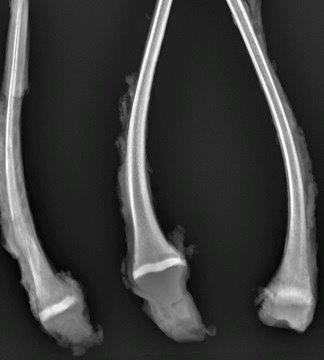
Gross Description:
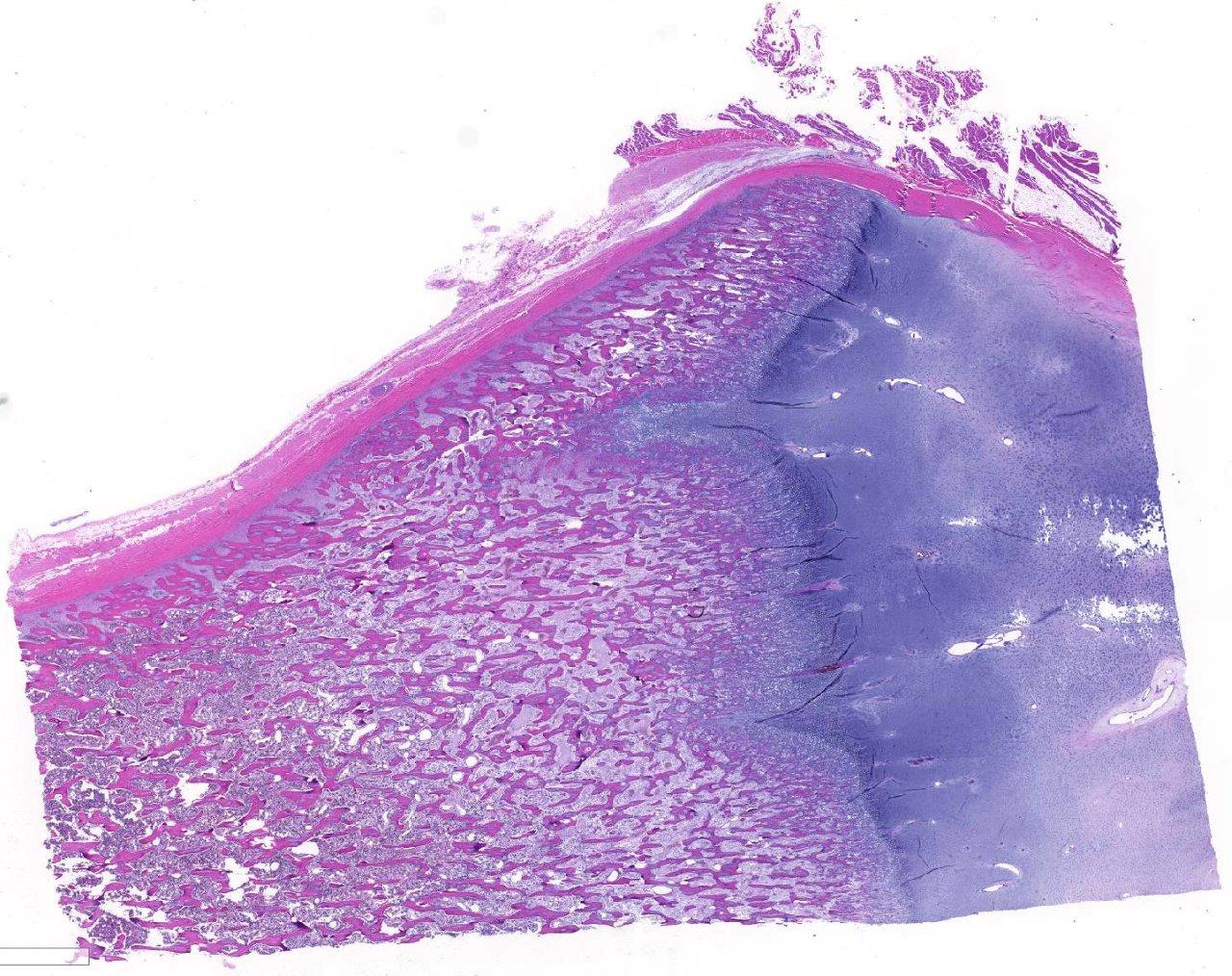
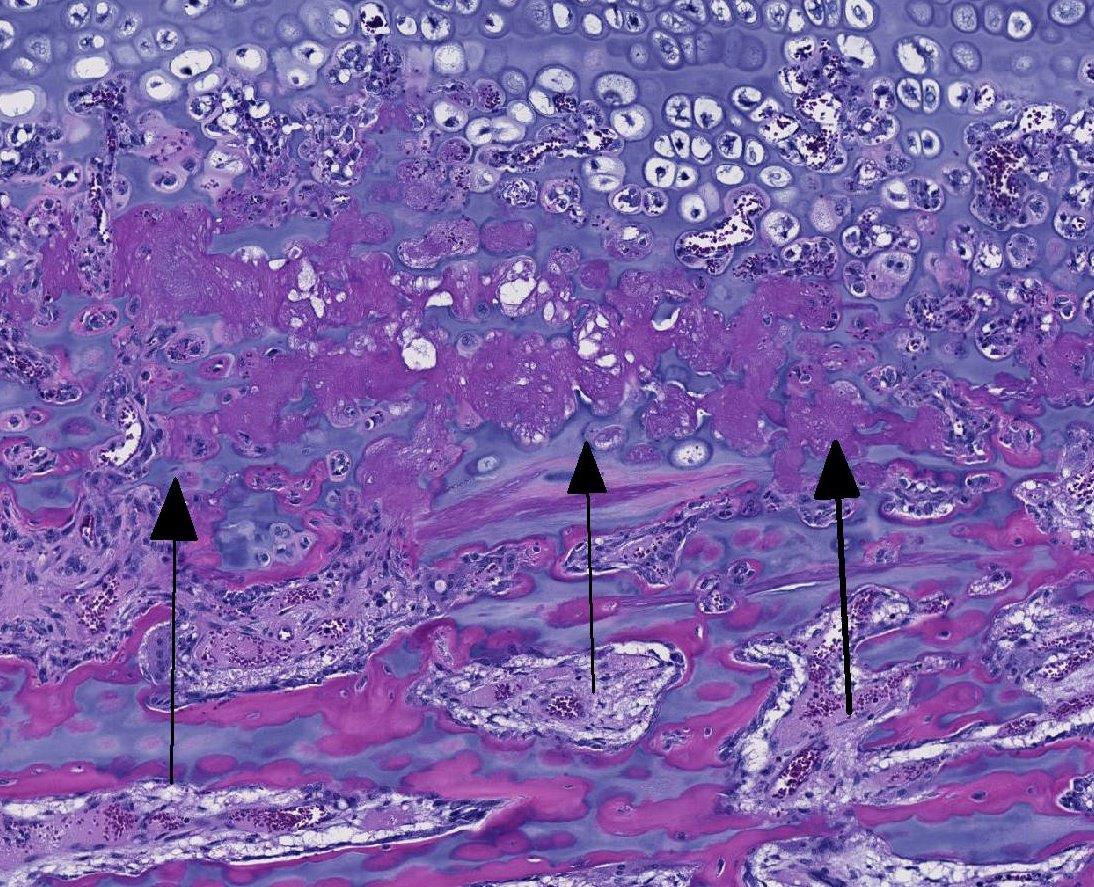
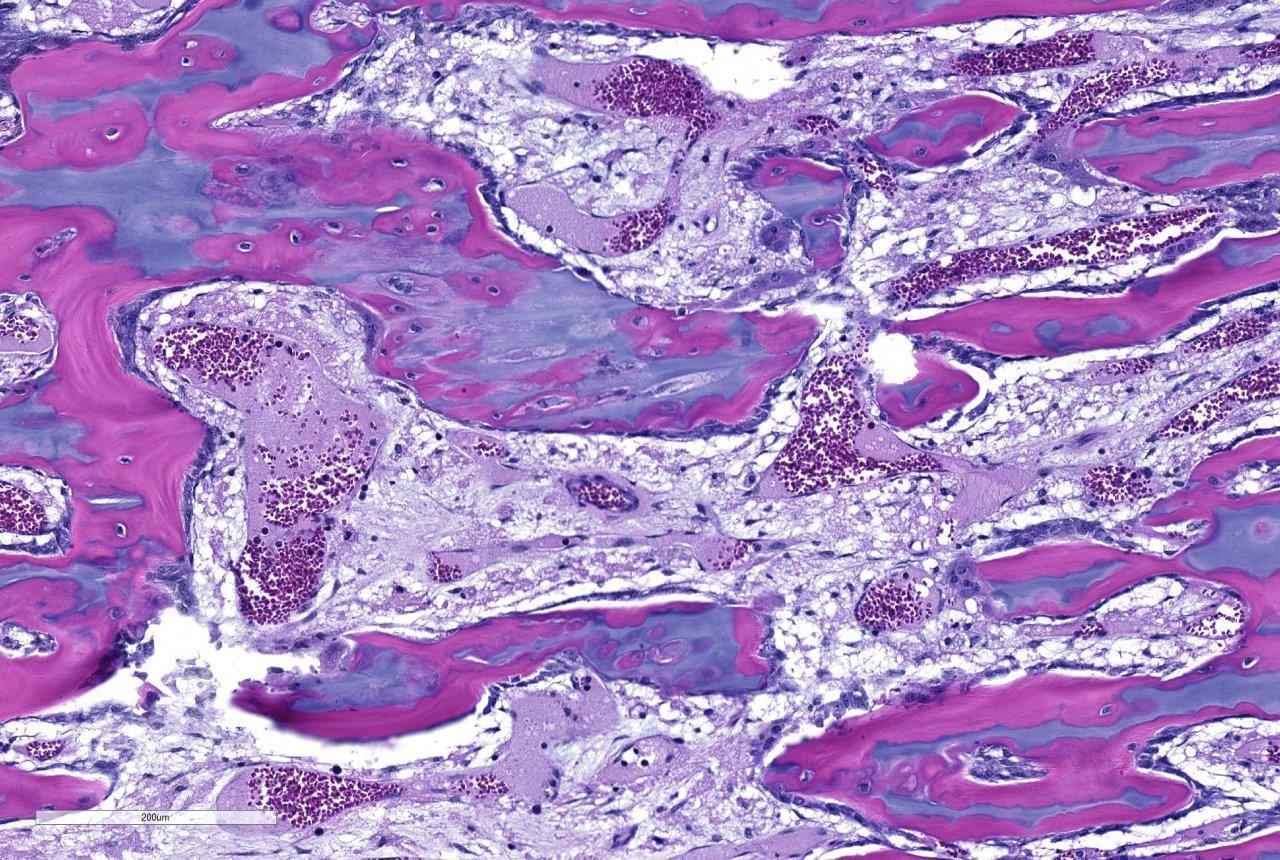
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
The bone lesions of lead poisoning were well described in the early 1930s by several authors studying poisoned children, and those descriptions remain valid. (Reviewed by Park 1933). The lead line in children will form once blood lead is 70-80 ug/dl. One month after treatment with chelating agents, the line separates from the zone of proliferating chondrocytes, and it will disappear in 4 years (Sachs 1981). Later descriptions added electron microscopic findings indicating that osteoclasts often lost their ruffled border and were less intimate with the surfaces (Eisenstein 1975). Nuclear and cytoplasmic inclusions were seen with EM. The cytoplasmic inclusions increased in size in osteoclasts more distant from the physis (inclusions fused?). Ultimately, morphologists concluded there is an inability for chondroclasts/osteoclasts to remove metaphyseal calcified cartilage cores presumably because they cannot degrade (or excrete) it. They are thus constipated.(Eisenstein 1975) Interestingly, lead binds to osteocalcin to make a more compact molecule, and lead can cause a 40% increase of hydroxyapatite mineral over that bound with calcium (Dowd 2008). Might such modifications lead to indigestion- to continue the ANALogy (JFE)? The increase in osteoclasts is a compensatory hyperplasia. Lead poisoning-induced, osteoclast intra-nuclear inclusions are visible with electron microscopy (Hsu 1973). These inclusions of lead and protein aggregates are best-known in proximal renal tubules (where they make up 90% of the lead in kidneys), but are also in osteoclasts and less frequently in hepatocytes and glia (Goyer 1970, Moore 1974). Experimental and spontaneous studies demonstrate they may appear and regress in intoxicated individuals (Hzu 1973, Hamir 1983, Goyer 1970). EM shows them more frequently, and they are seen occasionally in light microscopy using Ziehl-Neelsen acid-fast stain. We could not see them reliably with acid-fast or PAS staining. In experimental lead poisoning of dogs (Zook 1972), metaphyseal sclerosis with retention of cartilage trabeculae having more mineralized cartilage with increased numbers of large osteoclasts distal from the physis were seen.
Obviously, the lines are seen in young animals forming bones. Lead intoxication is more common in calves and thus are seen during calving season. Cattle usually have exposure to old lead base paints, discarded lead batteries, solder, linoleum, mining, smelting, and crankcase oil (ingested or used on skin as an insect repellant!) in pens or pastures (Blakley 1984, Burren 2010). Sometimes, recycling materials are incriminated (Payne 2008). Cases where pastures are previous shooting ranges have produced lead intoxications (Payne 2013). The decreased use of leaded gasolines has reduced risk of lead poisoning (Burren 2010). Blood, liver and kidney are favored samples to measure lead. When examining blood, many animals having measurable blood lead will not show signs (diarrhea, seizures, bruxism, blindness, hemorrhages). The half-life of lead in exposed cattle is 135 days, std deviation 125 days (Bischoff 2012, Voigt 2010). Our calf was ill and had laminar cortical necrosis. Unfortunately, a specific lead source in this case was not found, and a farm visit was not allowed. The potatoes mentioned in the history were never provided and are considered a red herring.
JPC Diagnosis:
Conference Comment:
This case generated spirited discussion among conference participants regarding whether the histologic lesions described in this case are consistent with lead intoxication. Conference participants described a diffusely thickened growth plate with multifocal tongues of cartilage cores extending into the metaphysis with a light blue to pink matrix and surrounded by a necrotic coagulum. Participants also noted few large vacuolated osteoclasts containing up to thirty nuclei within Howships lacuna but were not able to identify intranuclear or intracytoplasmic inclusions noted by the contributor.
Prior to the conference, the moderator, Dr. Linden Craig, examined the long bones of several age-matched control calves without lead intoxication. The consensus opinion of the conference moderator and participants is that there is no significant difference between the amount of mineralized cartilage trabeculae in the calf from this case and an aged matched control animal. This represents a disconnect between the sclerotic metaphysis seen both grossly and radiographically in this case, and the histologic appearance which lacks the significant retention of mineralized cartilage trabeculae within the metaphysis when compared to the rib of age-matched control. Some participants posited that this may be an artifact of decalcification processing of this section. Without the aid of the gross, radiographic, and historical data, diagnosis of lead intoxication in this case is extremely difficult.
References:
1. Bischoff K, Thompson B, Erb HN, Higgins WP, Ebel JG, Hillebrandt JR. Declines in blood lead concentrations in clinically affected and unaffected cattle accidentally exposed to lead. J Vet Dign Invest. 2012; 24:182-7.
2. Blakley BR. A retrospective study of lead poisoning in cattle. Vet Hum Toxicol. 1984; 26: 505-7.
3. Burren BG, Reichmann KG, McKenzie RA. Reduced risk of acute poisoning in Australian cattle from used motor oils after introduction of lead-free petrol. J Aust Vet Asso. 2010; 88: 240-241.
4. Craig LE, Dittmer KE, ThompsonKG. Bones and joints. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier Ltd; 2016:16-87.
5. Dowd TL, Li L, Gundberg CM. The 1H NMR structure of bovine PB2-osteocalcin and implications for lead toxicity. Biochim Biophys Acta. 2008; 1784:1534-45.
6. Eisenstein R, Kawanoue S. The lead line in bon-A lesion apparently due to chondroclastic indigestion. Am J Pathol. 1975; 80: 309-16.
7. Goyer RA, May P, Cates M, Krigman MR. Lead and protein content of isolated intranuclear inclusion bodies from kidneys of lead-poisoned rats. Lab Invest. 1970; 22:245-251.
8. Hamir AN, Sullvan ND, Handson PD. Acid fast inclusions in tissues of dogs dosed with lead. J Comp Pathol. 1983; 93:307-17.
9. Hsu FS, Krook L, Shively JN, Duncan JR. Lead inclusion bodies in osteoclasts. Science. 1973; 181:447-8.
10. Moore JF, Goyer RA. Lead-induced inclusion bodies. Composition and probable role in lead metabolism. Environ Health Perspec. 1974; 7:121-7.
11. Olson EJ, Carlson CS. Bones, joints, tendons, and ligaments. McGavin MD,ed. Pathologic basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier Mosby; 2017:964-965.
12. Park EA, Jackson D, Goodwin TC, Kajdi L. X-ray shadows in growing bones produced by lead; Their characteristics, cause, anatomical counterpart in the bone and differentiation. J Pediatr. 1933; 3: 265-300.
13. Payne JH, Holmes JP, Hogg RA, van der Burgt GM, Jewell NJ, Welchman G de B. Lead intoxication from clay pigeon shooting. Vet Rec. 2013; 173:552-4.
14. Payne J, Otter A, Cranwell M, Jones J, Wessells M, Whitaker K. Lead poisoning associated with recycled wood products. Vet Rec. 162: 191-2.
15. Sachs HK. The evolution of the radiographic lead line. Radiol. 1981; 139: 81-85.
16. Thompson K: Bones and joints. In: Maxie MG ed. Jubb Kennedy and Palmers Pathology of Domestic Animals, 5th edition. Saunders Elsevier New York; 2007:53-54.
17. Voigt K, Benavides J, Rafferty A Howie F, Buxton D. Lead poisoning in calves with eosinophilic meningitis. Vet Rec. 2010; 167:791-2.
18. Zook BC. The pathologic anatomy of lead poisoning in dogs. Vet Pathol. 1972; 9, 310-327.