Signalment:
Adult female wild turkey (
Meleagris gallopavo).Several individuals within a single flock of turkeys reported to be exhibiting clinical signs, including falling out of the trees where they roost and circling on the ground. An unspecified number were found dead.
Gross Description:
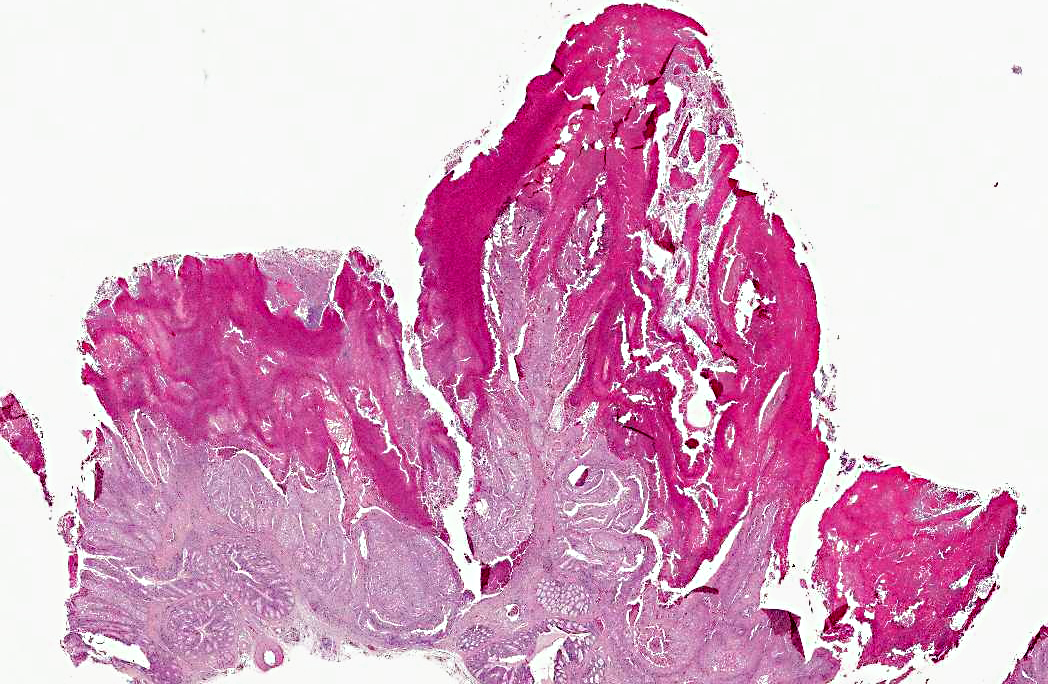
Within the oral cavity of affected animals, and largely effacing the normal mucosal surfaces, are numerous variably sized and irregularly shaped, multifocal to coalescing, proliferative, yellow-tan to pale yellow-green, caseous, nodular masses, which are variably covered in necrotic debris and fibrin.
Histopathologic Description:
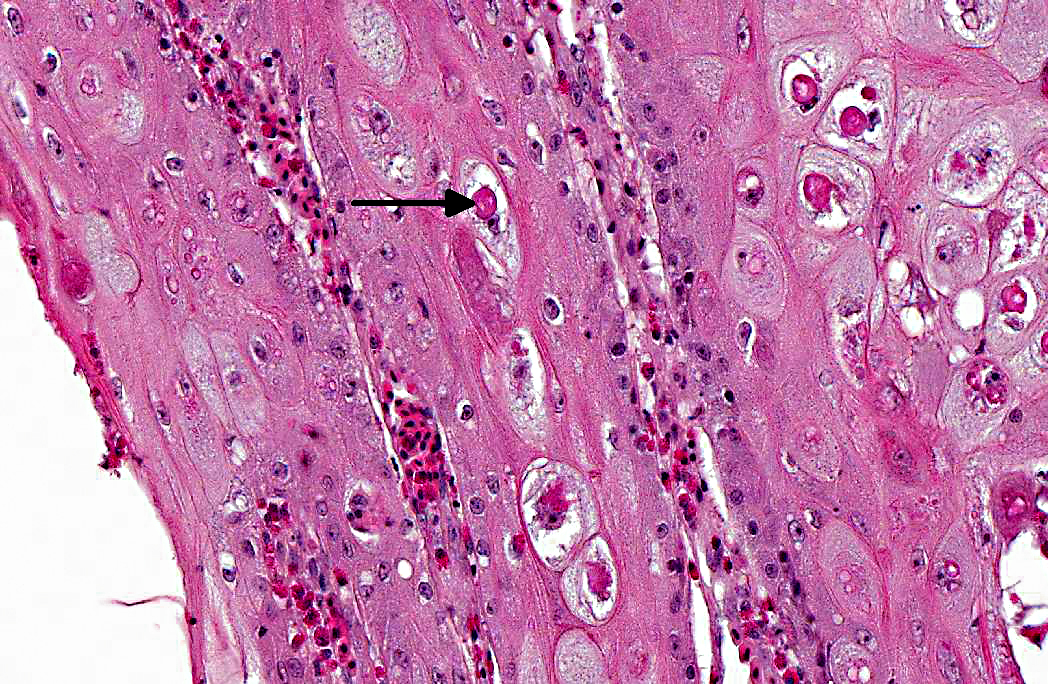
Oral cavity and tongue (sections vary): Within the sections examined are several areas of moderate to severe, locally to regionally extensive epithelial hyperplasia with occasional formation of variably sized and shaped papillary and frond-like projections. Many affected regions are characterized by variable degrees of erosion and superficial, adherent mats and masses of abundant amorphous eosinophilic necrotic material, cellular debris, blood, and occasional colonies of mixed bacteria. Individual epithelial cells in affected regions frequently have swollen, pale cytoplasm and there are many prominent, large, round to frequently ring-shaped, eosinophilic cytoplasmic inclusion bodies (Bollinger bodies). Affected cells also contain variably sized clear cytoplasmic vacuoles. Scattered individual cell necrosis is present. Similar hyperplastic and cytoplasmic changes occasionally extend into the epithelium of the underlying glandular tissue (not present in all slides). Within the underlying submucosal tissue is an inflammatory response composed predominantly of heterophils and macrophages, with smaller numbers of lymphocytes. Some sections contain deeper skeletal muscle, which contains rare, round to oval, intra-sarcoplasmic protozoal cysts characterized by a thin outer wall and abundant, central, crescent-shaped, basophilic bradyzoites (Sarcocystis).
Morphologic Diagnosis:
Oral cavity: Severe, multifocal to locally extensive, hyperplastic, necrotizing and erosive stomatitis, heterophilic and histiocytic, with epithelial ballooning degeneration and intracytoplasmic inclusion bodies, etiology consistent with avian poxvirus.
Condition:
Avian pox, diptheritic form
Contributor Comment:
The gross and histologic appearances of the lesions in the affected animals are typical of avian pox. This is a viral disease of birds that occurs nearly worldwide, with the exception of the polar regions. The avipoxviruses consist of a large, incompletely described group of viruses that affect both domestic and wild birds, and exhibit significant variability in host-specificity.(1,5,8) Poxviral disease has been reported in up to 278 species of birds and across at least 20 orders, and tends to be more prevalent in warmer and temperate regions of the globe.8 At this time, the International Committee on Taxonomy of Viruses only recognizes 10 distinct avipoxviruses, but 3 other viruses are tentatively recognized, and there are many additional viruses that have not yet been fully characterized.(9) The primary source of infection is infected birds, and transmission occurs both by means of biting insects serving as mechanical vectors, and through direct contact.(8)
Clinical disease in all affected birds occurs in two main forms: the cutaneous form, in which multiple nodular and proliferative lesions develop on the skin, and the diphtheritic form, which is characterized by extensive necrotizing foci in the oral cavity and upper respiratory tract.(8) The diphtheritic form is less commonly observed, especially in wild birds, but frequently results in increased morbidity and mortality. This particular case represents an example of the diphtheritic form.
Avipoxviruses are large, oval-shaped viruses with a double stranded DNA genome. Due to their large size (up to 250 by 350 nm), and unlike other DNA viruses, poxviruses replicate in the cytoplasm of infected cells where they induce a cytopathic effect and form characteristic eosinophilic inclusion bodies (Bollinger bodies).(9) Avipoxviruses have classically been viewed as highly species-specific, but there is great variability in this, with some isolates inducing severe disease in non-host species, and other isolates causing little to no clinical disease.(6,9)
In North American wild turkeys, poxviral infection has been observed, and is the most commonly diagnosed viral disease in several states, but is geographically centered on the southeastern portion of the United States.(2,3) One report describes a localized outbreak within a single flock in Oregon, but in general this disease is not frequently observed in western or northern states.(7) This particular outbreak is unusual in that it occurred in the northwestern region of the continent, during mid-winter, when insect vectors are at their lowest numbers. Given these considerations, there may have been some other point source of infection, potentially including contact with infected domestic poultry.
Further diagnostics to specifically identify the virus within these affected animals were not performed. As previously mentioned, avipoxviruses have traditionally been regarded as highly species specific, and clinical disease in turkeys (wild or domestic) would be expected to be caused by infection with turkeypox virus. However, a recent report describes infection of turkeys with the closely related fowlpox virus, calling the extent of species-specificity into question and raising the possibility that this particular outbreak may also have been caused by fowlpox virus or even another closely related virus.(5,6)
JPC Diagnosis:
Oral cavity: Stomatitis, necrotizing and proliferative, multifocal, marked, with ballooning degeneration and eosinophilic intracytoplasmic viral inclusion bodies.
Conference Comment:
The contributor provided a very good summary of avian poxviruses. Viruses in the family
Poxviridae are epitheliotropic DNA viruses that cause cutaneous or systemic disease in many species of animals, including wild and domestic mammals, birds, and humans. Poxviruses induce their characteristic lesions of epidermal hyperplasia, ballooning degeneration and vesicular lesions through several mechanisms. Many poxviruses encode a gene whose product is analogous to epidermal growth factor, and whose stimulation of host cell DNA results in epidermal hyperplasia. Vascular damage (due to viral multiplication in endothelial cells) and epidermal hyperplasia can result in ischemic necrosis and subsequent degenerative lesions in dermal and submucosal tissues.(7) Another characteristic feature of poxvirus-infected cells is the presence of one or more, variably-sized, intracytoplasmic eosinophilic viral inclusions that are composed predominantly of proteins. In addition to the previously-described genes, poxviruses also contain genes that encode proteins to circumvent the hosts defense systems. One such protein is closely related to the superfamily of proteins known as SERPINS, which act as regulators of serine protease enzymes that mediate kinin, complement, fibrinolytic and coagulation pathways, thus allowing the poxviruses to inhibit host defenses. Additional poxvirus genes encode proteins that have anti-interferon activities, further rendering the host defenses inadequate against the virus. Pox lesions generally develop in a typical sequence, beginning as erythematous macules, which become papules, then progress to vesicles, which further develop into pustules which ultimately rupture and become umbilicated with a characteristic depressed center and raised, erythematous border (the pock). Once the pustules rupture, a crust forms and healed lesions often leave scars. Mucosal lesions often develop into ulcers rather than pustules. In addition to these cutaneous lesions, some poxviruses (such as Sheeppox virus, Ectromelia virus, Monkeypox virus, and Variola virus) also cause severe systemic disease. Following is a list of important poxviruses in veterinary medicine:(7)
| Genus | Poxvirus |
| Orthopoxvirus | Camelpox virus, Cowpox virus, Ectromelia virus (mousepox), Monkeypox virus, Vaccinia virus (buffalopox virus, rabbitpox virus), Uasin Gishu disease virus* |
| Parapoxvirus | Bovine popular stomatitis virus, Contagious ecthyma virus (Orf virus), Parapox virus of red deer, Pseudocowpox virus, Auzduk disease virus* (Camel contagious ecthyma virus), Chamois contagious ecthyma virus*, Sealpox virus* |
| Avipoxvirus | Fowlpox virus, Pigeonpox virus, many other avian poxviruses |
| Capripoxvirus | Goatpox virus, Lumpy skin disease virus, Sheepox virus |
| Leporipoxvirus | Myxoma virus, Rabbit fibroma virus (Shope fibroma virus) |
| Suispoxvirus | Swinepox virus |
| Molluscipoxvirus | Molluscum contagiosum virus |
| Yatapoxvirus | Tanapox virus, Yaba monkey tumor virus |
*Unassigned members of the genus
7
References:
1. Bolte AL, Meurer J, Kaleta EF. Avian host spectrum of avipoxviruses.
Avian Pathol. 1999;28:415-432.
2. Davidson WR, Nettles VF, Couvillion E, et al. Diseases diagnosed in wild turkeys (
Meleagris gallopavo) of the southeastern United States.
J Wildl Dis. 1985;21(4):386-390.
3. Forrester DJ. The ecology and epizootiology of avian pox and aalaria in wild turkeys.
Bull Soc Vector Ecol. 1991;16(1):127-148.
4. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed.
Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed. New York, NY: Saunders Elsevier; 2007:664-665.
5. Hess C, Maegdefrau-Pollan B, Bilic I, Liebhart D, et al. Outbreak of cutaneous form of poxvirus on a commercial turkey farm caused by the species fowlpox.
Avian Dis. 2011;55:714-718.
6. Jarmin S, Manvell R, Gough RE, et al. Avipoxvirus phylogenetics: identification of a PCR length polymorphism that discriminates between the two major clades.
J Gen Virol. 2006;87:2191-2201.
7. Lutz RS, Crawford JA. Prevalence of poxvirus in a population of Merriams Wild Turkeys in Oregon.
J Wildl Dis. 1987;23(2):306-307.
8. Van Riper III C, Forrester DJ. Avian pox. In: Thomas NJ, Hunter DB, Atkinson CT, eds.
Infectious Diseases of Wild Birds. Ames, IA: Blackwell Publishing; 2007:131-176.
9. Weli SC, Tryland M. Avipoxviruses: infection biology and their use as vaccine vectors.
Virology Journal. 2011;8(49):1-15