Wednesday Slide Conference, Conference 21, Case 3
Signalment:
1-year-old male intact standard fancy rat (Rattus norvegicus domestica)
History:
Submitted for necropsy is the body of a 1-year-old male intact pet rat adopted from a rescue a few months prior, with a reported history of waxing and waning nodular facial swelling located under the right eye, followed by diffuse facial swelling. This progressed to upper respiratory signs that resolved with enrofloxacin. The facial swelling was treated with a course of prednisolone and meloxicam. After a period of treatment, the signs worsened, characterized by a new eruption of multiple firm nodules near the nasal angle of the eyes and over the snout with increased respiratory distress. After 48h of increased respiratory rate and effort along with difficulty swallowing, the patient was euthanized due to poor prognosis.
Gross Pathology:
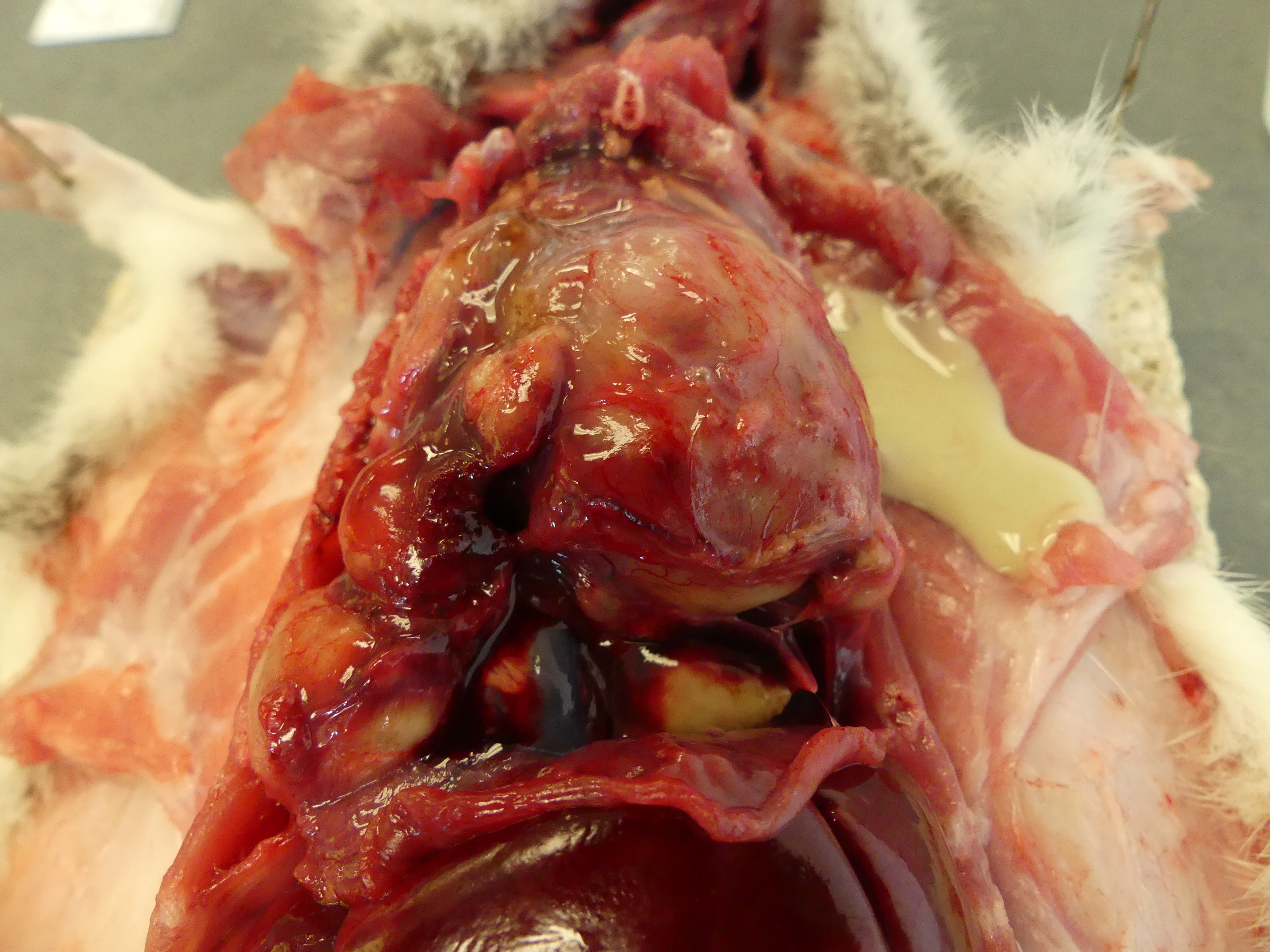
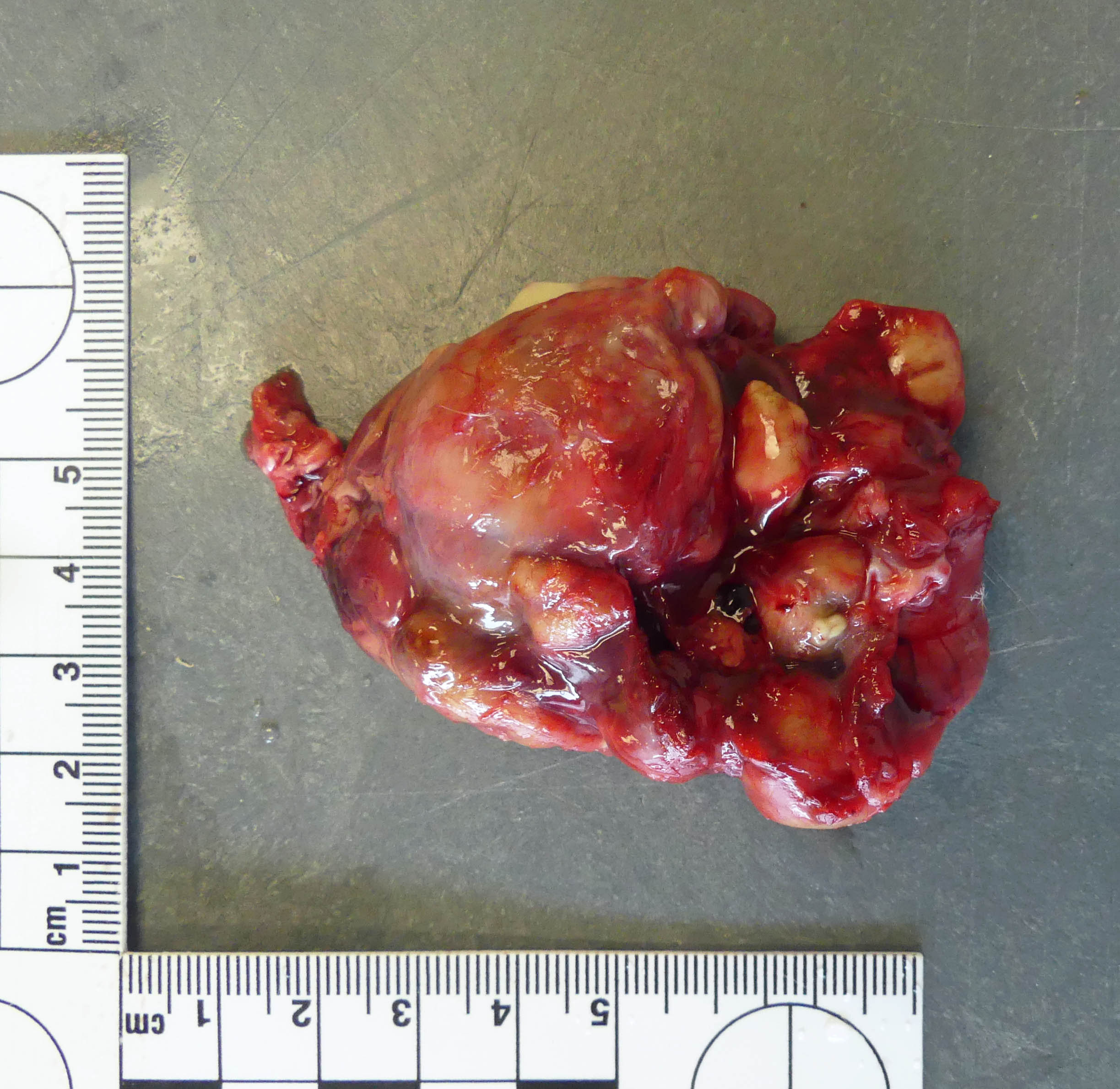
The lungs are entirely replaced, severely deformed, and expanded by over three dozen variably sized, up to 3.8 x 2.4 x 1.8 cm, multifocal to coalescing spherical, tan masses, rendering a bosselated appearance to the parenchyma, in particular of the right lung. On cut section, the masses extend deep within the parenchyma and exude abundant thick, opaque, light-yellow to white, malodorous pasty material (suppurative pneumonia with bronchiectasis). Additionally, the pleura firmly adheres multifocally to the diaphragm and thoracic wall, and the heart is obscured by a pale pink mass of suppurative material and fibrosis (pericardial abscess).
Laboratory Results:
Bacterial culture was performed of the lungs, which isolated both Mycoplasma sp. and Rodentibacter pneumotropicus. Further speciation of Mycoplasma sp. was not conducted. Exclusion of Filobacterium rodentium was initially desired; unfortunately, the specific type of culture medium for this agent was not available at the testing facility.
Microscopic Description:
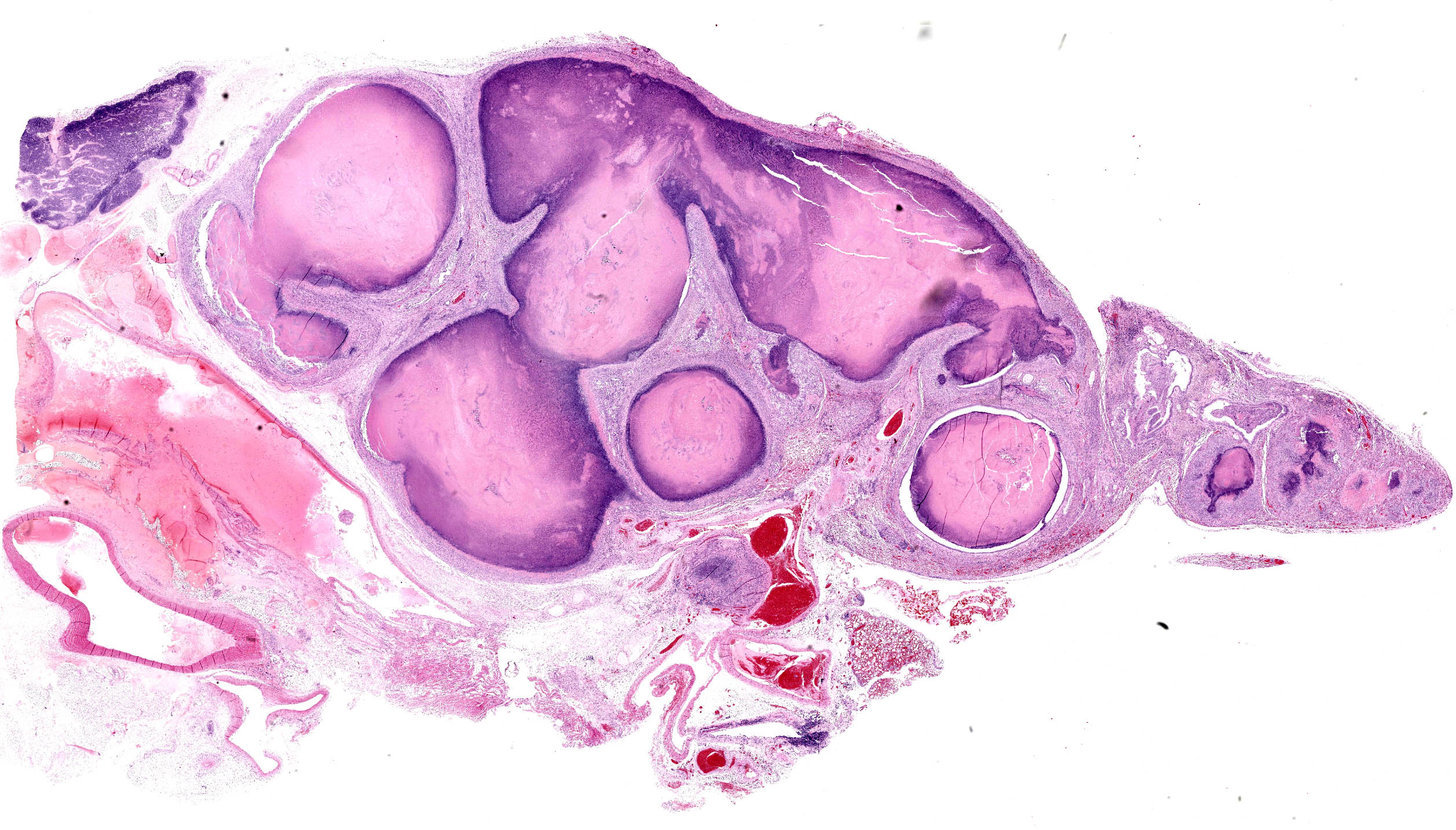
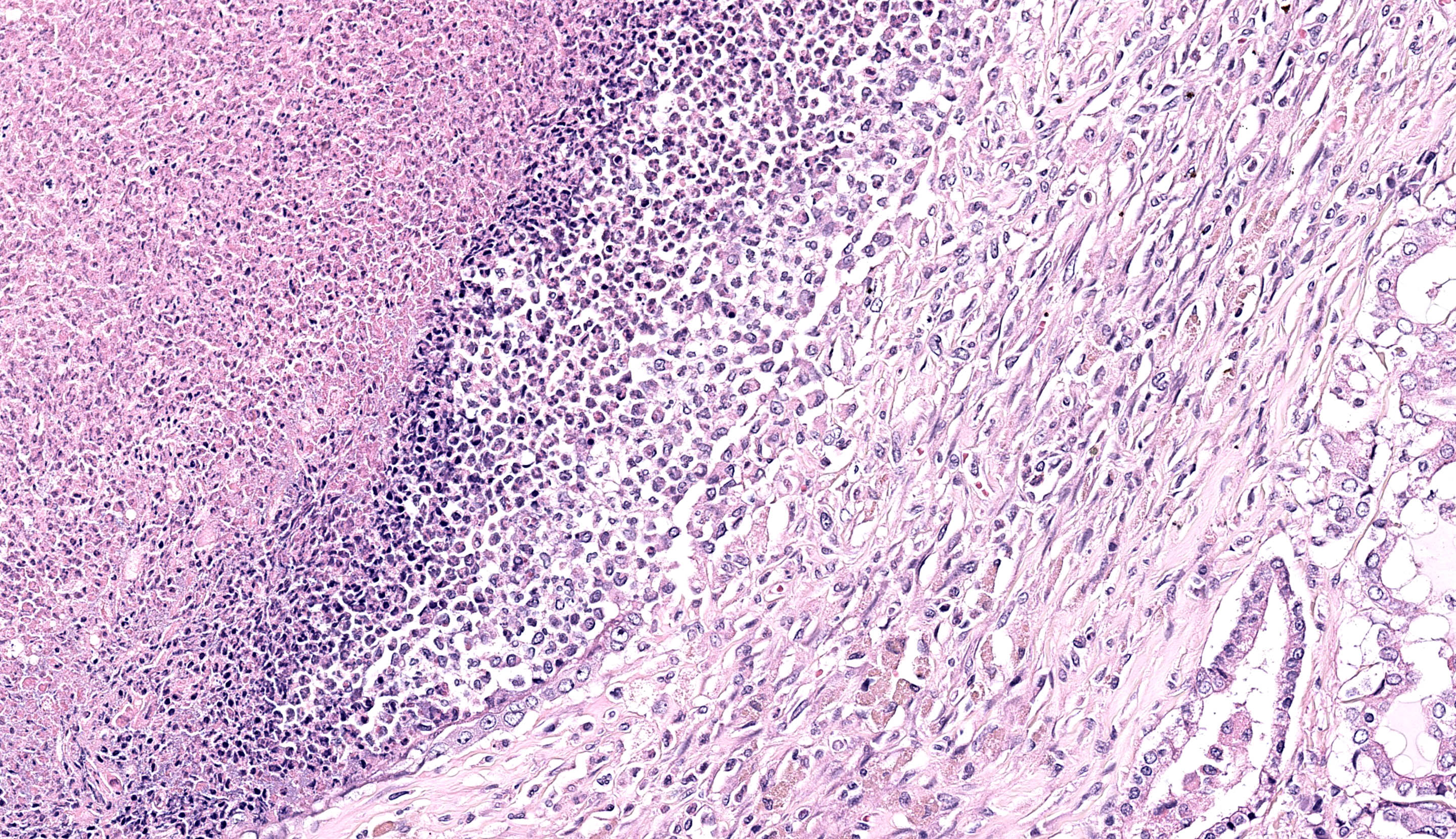
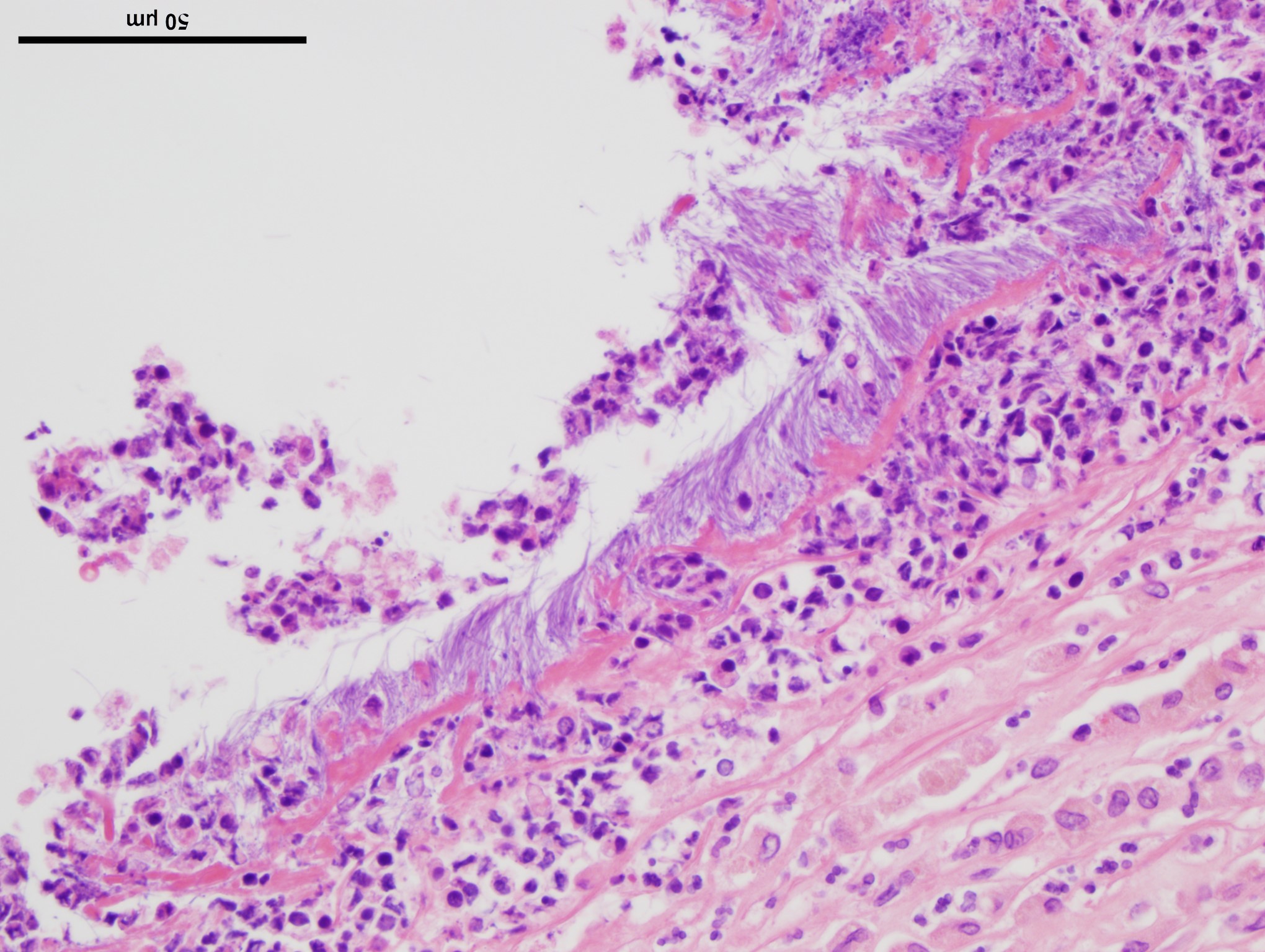
Lung: Up to 85% of the parenchyma in the submitted sections is effaced and replaced by variable numbers of well-delineated, coalescing spherical nodules arising within severely ectatic pre-existing bronchi (bronchiectasis), and enclosed in a thick fibrous capsule stippled with admixed lymphocytes, plasma cells, and macrophages, along with scattered stout congested capillaries. These nodules are mostly composed of central, compact amorphous eosinophilic material, vaguely imprinted with leukocytic debris, and rimmed by abundant degenerate neutrophils. The bronchial epithelium lining the areas of bronchiectasis is circumferentially replaced by an up to 10 cells-thick, keratinizing stratified squamous epithelium (squamous metaplasia). Covering this epithelium and projecting towards the lumen are mats of up to 18.0um-long and 1.0um thick, faintly amphophilic to eosinophilic filamentous bacteria.
Remnant alveoli within the surrounding parenchyma are compressed, distorted and completely filled with degenerate neutrophils, lymphocytes, plasma cells and foamy macrophages, embedded within variable quantities of light eosinophilic, proteinaceous fluid (pulmonary edema). The alveolar lining is extensively replaced by a continuous single cell-thick, low-cuboidal epithelium (pneumocytes type II hyperplasia). When appreciable, bronchial glands are prominent, mildly distorted and hyperplastic. Variably throughout the sections, bronchioles and bronchi are severely cuffed by abundant admixed lymphocytes and plasma cells, equally encircling infrequently thrombosed pulmonary arterioles.
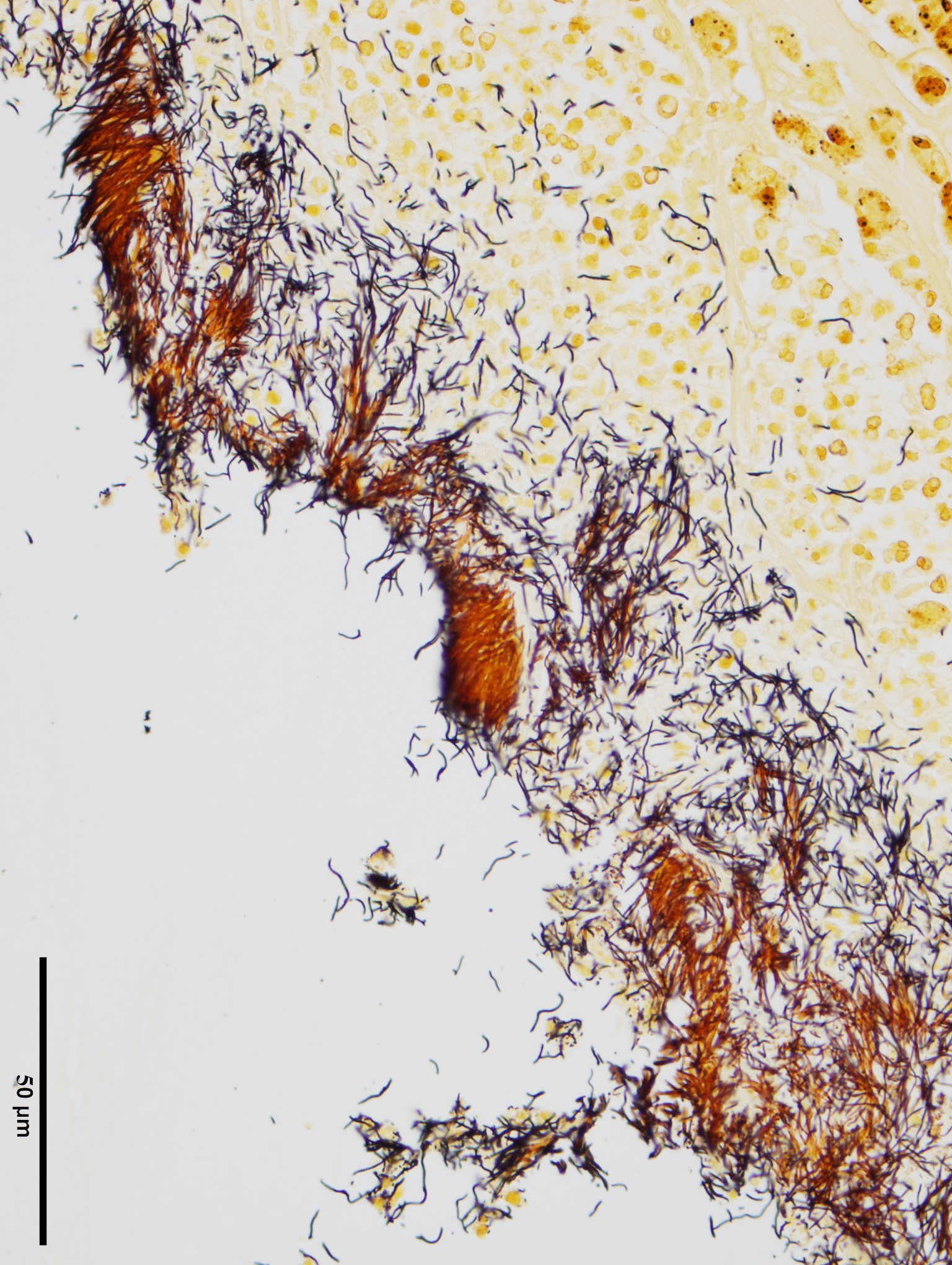
Two additional stains, modified Steiner and Gram, were applied to a section of lung. The modified Steiner highlighted a thick mat of up to 18.0 μm-long, free filamentous argyrophilic bacteria corresponding to the organisms appreciated on hematoxylin eosin, disposed perpendicularly to the metaplastic bronchial epithelium. Thousands of identical free bacteria were distributed throughout the suppurative material filling the ectatic bronchi.
The Gram stain highlighted similar Gram-negative, filamentous bacteria throughout the sections, covering the metaplastic bronchial epithelium and interspersed with sparsely scattered Gram-negative, 0.3 μm-long coccobacilli. Clusters of approximately 0.2 μm in diameter, Gram-positive coccobacilli were additionally dispersed throughout the suppurative material.
Contributor’s Morphologic Diagnosis:
Lung: Severe, multifocal to coalescing, chronic suppurative bronchopneumonia with bronchiectasis and myriad intralesional filamentous bacteria, lymphoplasmacytic peribronchiolitis and perivasculitis, squamous metaplasia of airways epithelium, glandular hyperplasia, type II pneumocyte hyperplasia, thrombosis and edema
Contributor’s Comment:
Grossly, this case was unusually extensive with regards to the diffuse bilateral involvement of the pulmonary parenchyma, although the lesions remained more severe within the right lung, consistent with the classic unilateral, cranio-ventral lesional distribution commonly encountered with Mycoplasma pulmonis.
Isolation of Mycoplasma sp. together with bronchiectasis allowed for a diagnosis of murine respiratory mycoplasmosis, also termed chronic respiratory disease (CRD). CRD is usually considered subsequent to the combined action of Mycoplasma pulmonis and other respiratory pathogens such as Filobacterium rodentium (formerly known as CAR bacillus),4 viral pathogens such as Sendai virus and rat coronavirus, and/or environmental causes such as high levels of ammonia.1,3
Mycoplasma pulmonis is directly deleterious to the respiratory epithelium, and is considered the only clinically relevant species of Mycoplasma in rodents.1,3 Transmission occurs vertically or via aerosols, although the latter has experimentally proven fairly inefficient.1,8 Colonization of the respiratory tract by Mycoplasma sp. results in ciliostasis and loss of cilia, subsequently reducing the efficacy of the mucociliary escalator, and caus-ing mucus and inflammatory exudate accumulation within the airways. Ultimately, the ongoing buildup of lysozyme-rich inflammatory material culminates in permanent damage and dilatation of airways, resulting in bronchiectasis.1,3 Both Mycoplasma microorganisms and host cell membrane fragments nonspecifically stimulate B-cell mitosis, and the resulting florid peribronchiolar lymphocytic infiltration is a salient histological feature of CRD in rats.1 Other respiratory manifestations of mycoplasmosis in rats include rhinitis and lymphoplasmacytic tracheitis.1,8
Given the tropism of M. pulmonis for ciliated epithelia, colonization and consequent inflammation of non-respiratory tissues, including the middle ear, endometrium and synovium, may also occur.1,3 M. pulmonis is usually not evident on hematoxylin eosin sections, and requires ancillary tests such as culture or PCR for confirmation.
Additionally in this rat, the myriad amphophilic to light basophilic filamentous bacteria appreciated lining the inside of ectatic bronchi were considered compatible with Filobacterium rodentium, formerly known as Cilia-Associated Respiratory (CAR) bacillus.4 F. rodentium is mostly transmitted by close contact during the neonatal period, and most strains of laboratory rats have been shown uniformly susceptible to disease.1,3 While M. pulmonis and F. rodentium co-infection is frequent, both may individually cause illness and result in similar clinical disease and histological lesions.1,3,8
Histologically, F. rodentium is best highlighted using silver stains (such as Warthin-Starry), and characteristically forms mats of filamentous bacteria colonizing the respiratory airways, interspersed with cilia and oriented in a relatively perpendicular fashion to the epithelium.1 F.rodentium has been isolated from the respiratory epithelium of other species than rat, including mice, rabbits, cattle, goats and pigs, and a recent report suggests that a particular strain of Filobacterium sp. could be associated with chronic bronchitis in cats.1,5
In the present case, these argyrophilic bacteria were emphasized using modified Steiner’s stain, which additionally revealed a significant number of those microorganisms within the suppurative material filling the airways. On Gram stain, the organisms appeared faintly Gram-negative. As mentioned in the above laboratory results section, confirmation by culture was unavailable as F.rodentium requires specific growth media,1,7 and this remains presumptive based on the combined histomorphological features, staining characteristics, and common reported co-involvement of M. pulmonis and F.rodentium in CRD.
Large numbers of Rodentibacter pneumotropicus were additionally isolated via lung culture. Formerly known as Pasteurella pneumotropica,2 this Gram-negative, 0.5 to 1.2 μm-long rod to coccobacillus is a resident bacterium of the respiratory flora in rats,1,3 and was thus considered an opportunistic co-infectious agent in the present case. Although not isolated on culture, clusters of Gram-positive bacteria were histologically appreciated; Gram-positive organisms involved in respiratory disease and inducing formation of abscesses in rats include Streptococcus pneumoniae and Corynebacterium kutscherii, the latter capable of causing cutaneous abscesses.1,3
Additional findings, not present in the submitted slides, include the presence of nematode parasites within the renal pelvis, considered consistent with Trichosomoides crassicauda, a roundworm that commonly invades the murine urinary tract in individuals housed in poor hygienic conditions.6 About five deep subcutaneous abscesses were additionally noted throughout the skin of the muzzle, with no identifiable intralesional bacteria. This was considered most probably consecutive to dissemination from the lungs in a relatively immunocompromised animal, given the absence of florid lymphoid hyperplasia in the present case, usually expected with Mycoplasma sp. infection.
Contributing Institution:
Cornell University College of Veterinary Medicine – Animal Health Diagnostic Center, Department of Biomedical Science, Section of Anatomic Pathology
https://www.vet.cornell.edu/animal-health-diagnostic-center
JPC Diagnosis:
Lung: Bronchopneumonia, suppurative, chronic, diffuse, severe, with bronchiectasis, fibrosis, and cilia-associated bacilli and luminal colonies of coccobacilli.
JPC Comment:
The third case of this conference has a terrific macropscopic-microscopic correlation.. We confirm the contributor’s interpretation of abundant argyrophilic filamentous bacteria with both Warthin-Starry and methenamine silver highlighting organisms within ectatic bronchioles well. We also noted a mixed population of bacteria on our Gram stain consistent with secondary opportunistic infection following ciliostasis initiated by Mycoplasma. Although subtle, there is also a change in appearance of ciliated epithelium with replacement of the normal eosinophilic hue towards a basophilic appearance of the cilia. Although non-specific, this is corroborating evidence of ‘something added’ such as Mycoplasma, Bordetella, or Filobacterium and can be seen even in sloughed epithelium. Although not stated by the contributor, we suspect that this animal likely had concurrent Mycoplasma in the ear to explain the facial signs observed.
Conference participants also discussed the presence of “oat cells” (ruptured leukocytes with streaming nuclear contents) which were a significant feature of this case. Oat cells have been described secondary to leukotoxins, a type of repeats in toxin (RTX) which is an important virulence factor for several bacterial pathogens including Mannheimia, Pasteurella, and Bordetella among others.11 Leukotoxins can enhance both recruitment and killing of leukocytes which confers an advantage in both hampering the host immune response and extracting cellular resources for future growth. Rodentibacter pneumotropicus (formerly Pasteurella pneumotropica) is a potential source of RTX toxin in this case,10 given the gram-negative coccobacilli we noted in section. Interestingly, lysis of leukocytes may be a double-edged sword, as extrusion of nuclear chromatin also enhances NETosis and may entrap and kill a portion of the bacteria concurrently.9
References:
- Barthold SW, Griffey SM, Percy DH, eds. Pathology of Laboratory Rodents and Rabbits. 4th ed. Ames, IA: Wiley-Blackwell; 2016: 133-136, 140-141, 144-145.
- Benga L, Sager M, Christensen H. From the [Pasteurella] pneumotropica complex to Rodentibacter spp.: an update on [Pasteurella] pneumotropica. Vet. Microbiol. 2018;217:121-134.
- Fox JG, Anderson LC, Otto GM, Prtichett-Corning KR, Whary MT, eds. Laboratory animal medicine. 3rd ed. San Diego, CA: Elsevier; 2015:166-169, 173-175.
- Ike F, Sakamoto M, Ohkuma M. et al. Filobacterium rodentium gen. nov., sp. nov., a member of Filobacteriaceae fam. nov. within the phylum Bacteroidetes; includes a microaerobic filamentous bacterium isolated from specimens from diseased rodent respiratory tracts Int. J. Syst. Evol. 2016; 66:150-157.
- Na?eradská M, Pekova S, Danesi P. et. A novel Filobacterium sp can cause chronic bronchitis in cats. PLOS ONE. 2021;16(6):e0251968.
- Najafi F, Ghadikolai MT, Naddaf SR. et al. Trichosomoides crassicauda infection in laboratory rats with histopathological description in the bladder tissue. J Med Microbiol Infec Dis. 2017;5(1-2):31-34.
- Nietfeld JC, Fickbohm BL, Rogers DG, Franklin CL, Riley LK. Isolation of cilia-associated respiratory (CAR) bacillus from pigs and calves and experimental infection of gnotobiotic pigs and rodents. J. Vet. Diagn. Invest. 1999;11:252-258.
- Rothenburger JL, Himsworth CG, Clifford CB, Ellis J, Treuting PM, Leighton FA. Respiratory Pathology and Pathogens in Wild Urban Rats (Rattus norvegicus and Rattus rattus). Veterinary Pathology. 2015;52(6):1210-1219.
- Aulik NA, Hellenbrand KM, Klos H, Czuprynski CJ. Mannheimia haemolytica and its leukotoxin cause neutrophil extracellular trap formation by bovine neutrophils. Infect Immun. 2010 Nov;78(11):4454-66.
- Sasaki H, Ueshiba H, Yanagisawa N, Itoh Y, Ishikawa H, Shigenaga A, Benga L, Ike F. Genomic and pathogenic characterization of RTX toxin producing Rodentibacter sp. that is closely related to Rodentibacter haemolyticus. Infect Genet Evol. 2022 Aug;102:105314.
- Srikumaran S. Leukotoxins. Toxins (Basel). 2020 Apr 7;12(4):231.