Signalment:
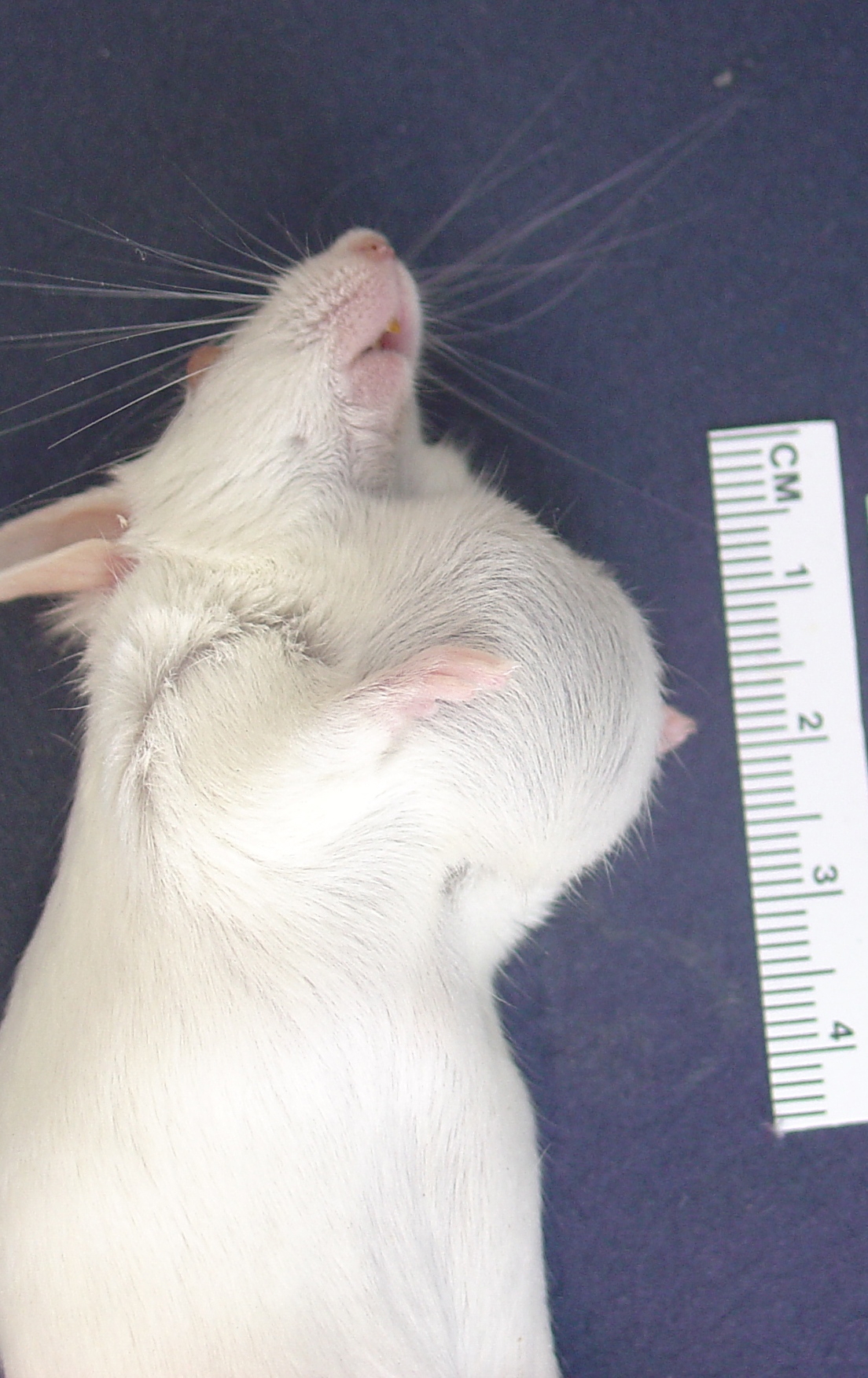
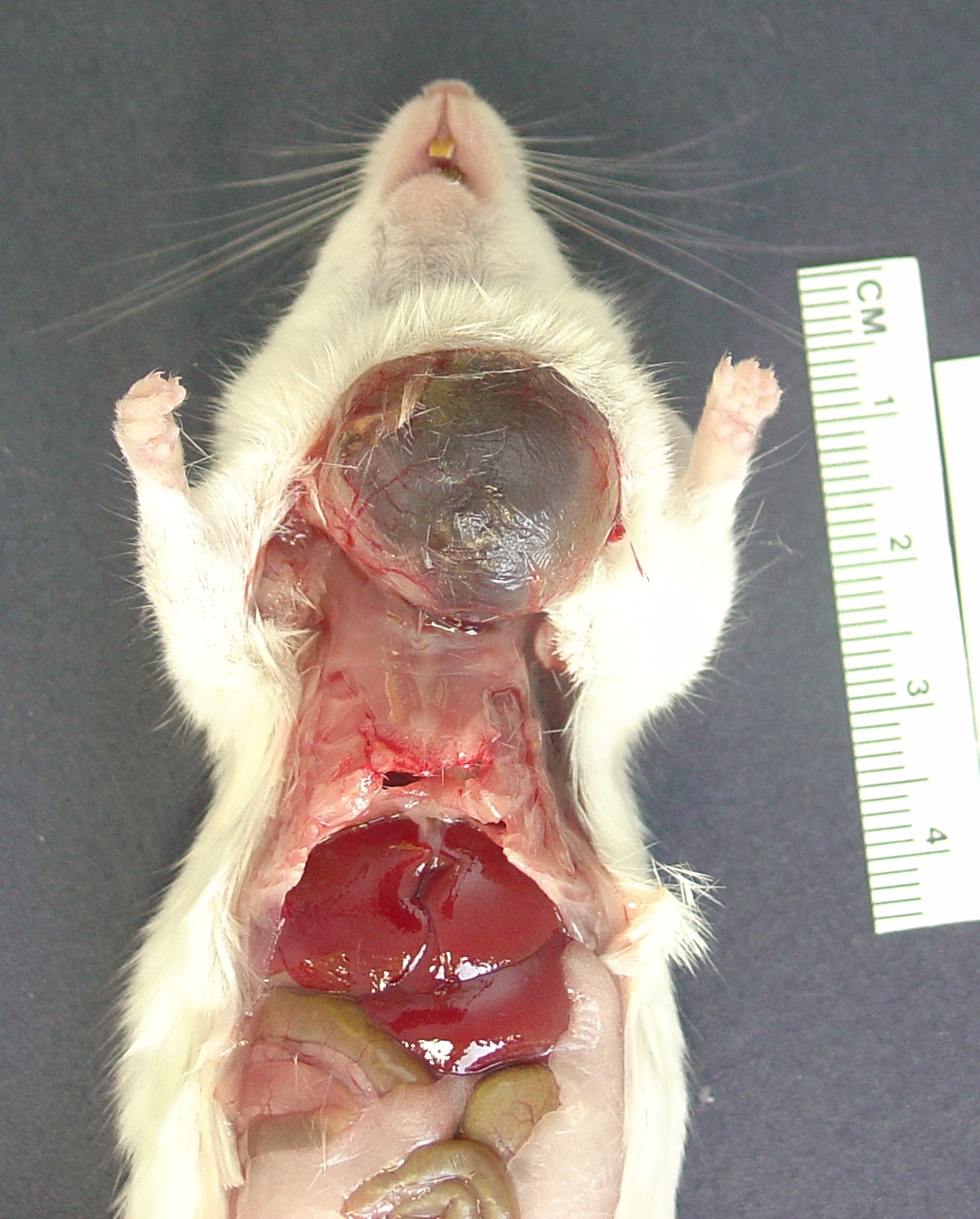
Gross Description:
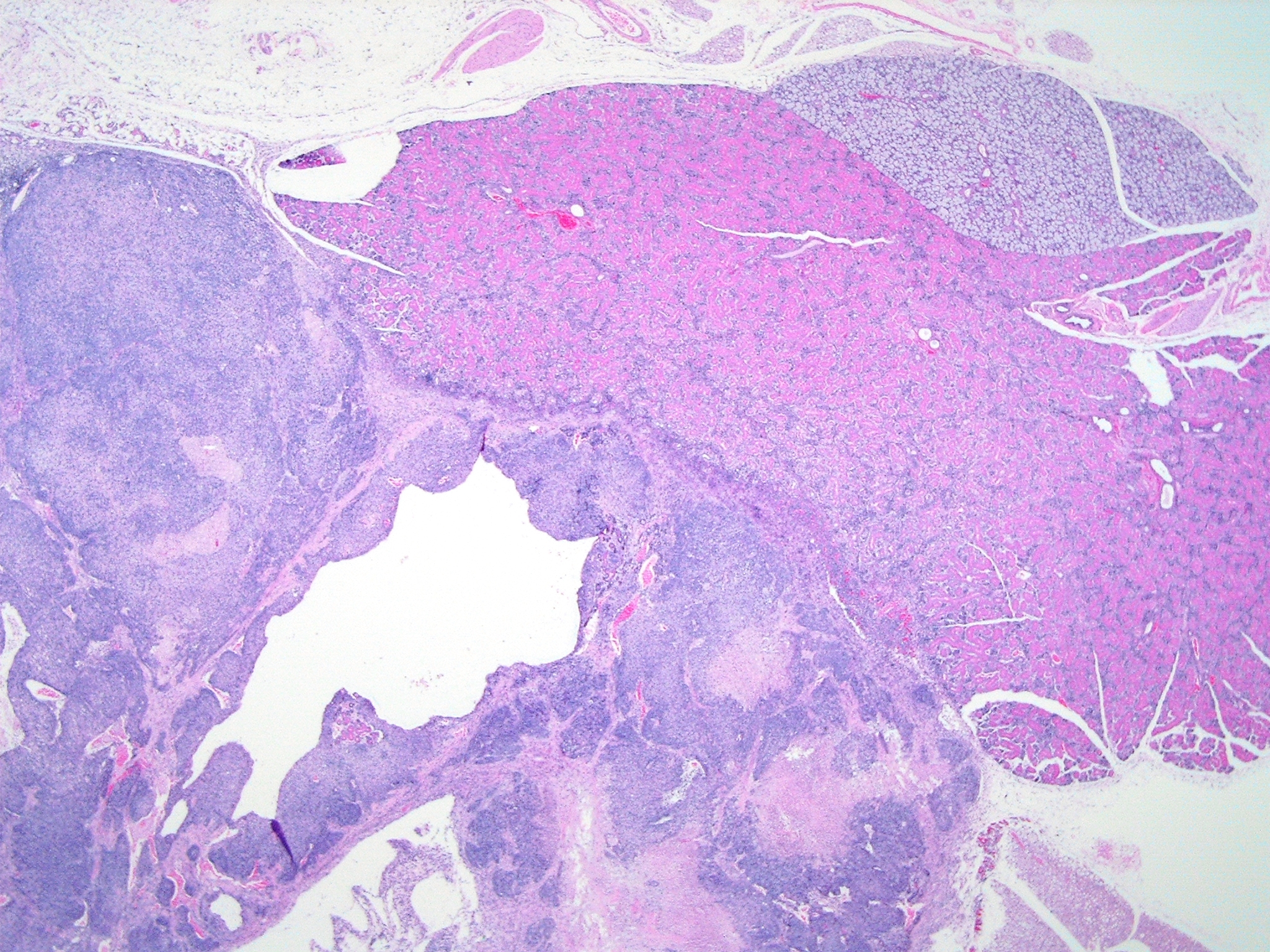
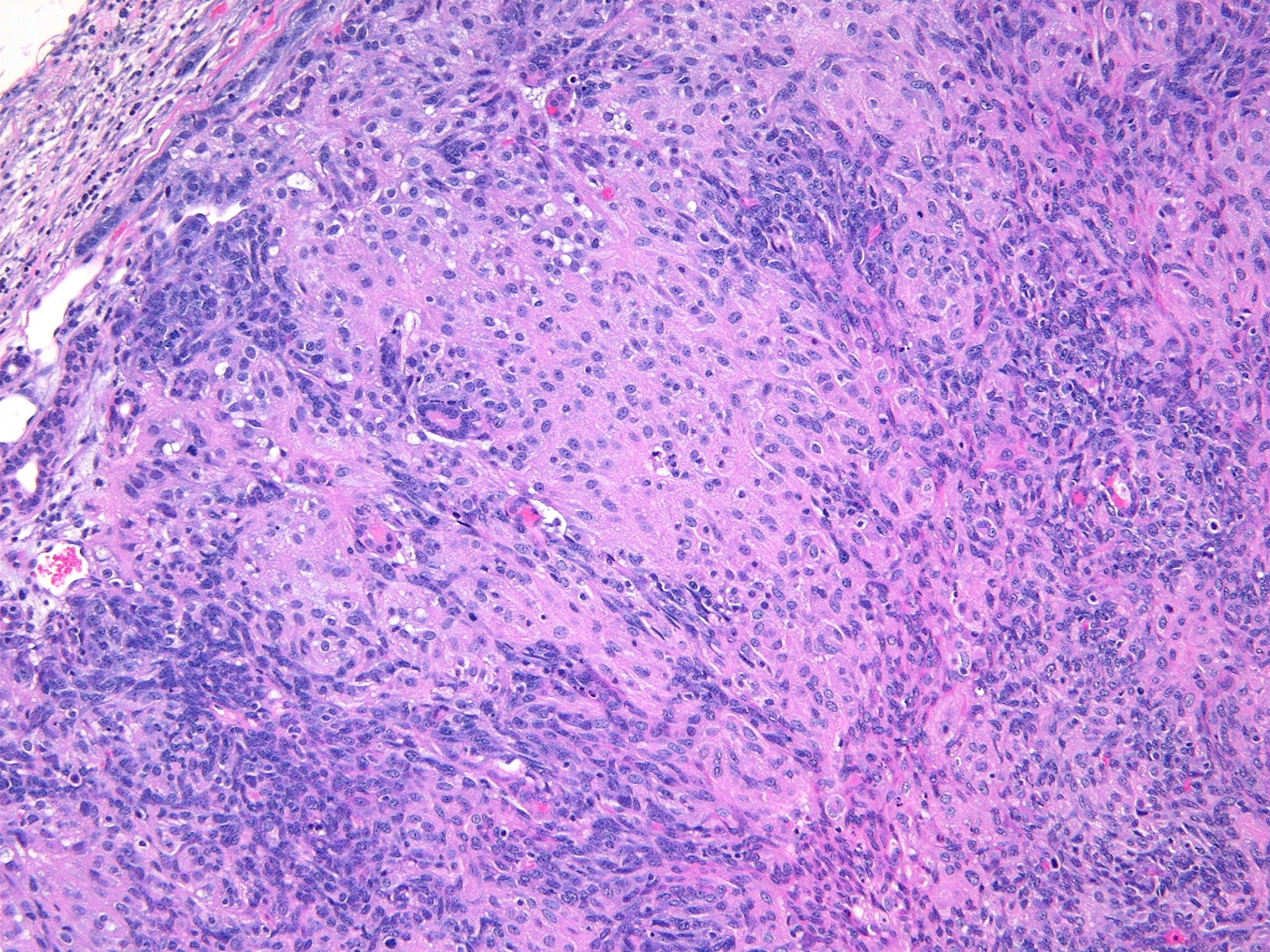
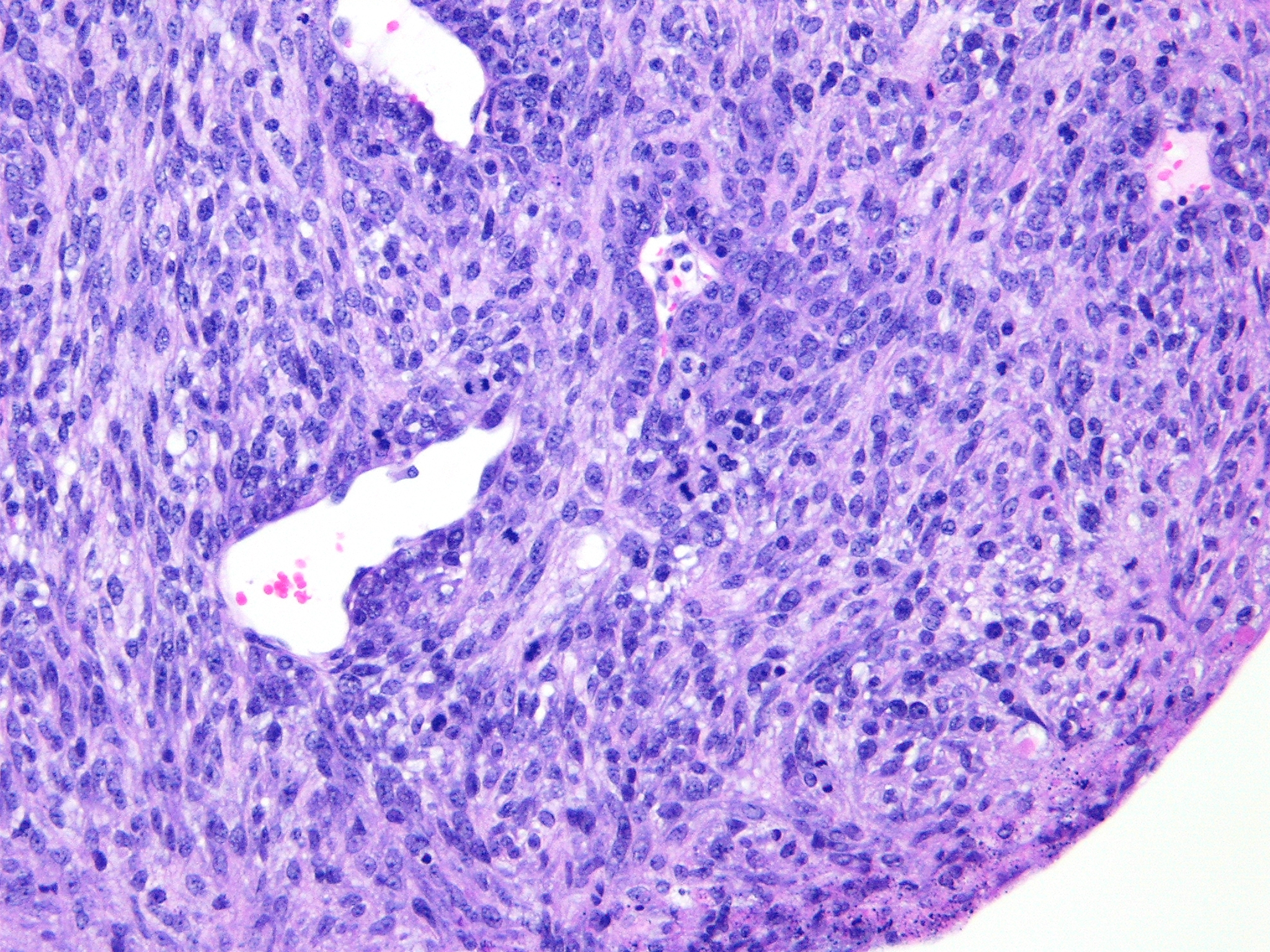
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
The characteristic absence of formation of acini or ducts by the neoplastic cells aids in differentiating these tumors from adenomas and carcinomas. The presence of a single solitary mass on the ventral aspect of the neck helps to differentiate these neoplasms from polyoma virus-induced pleomorphic tumors that are multicentric in origin and not limited to the salivary glands.(3) Myoepitheliomas can also occur within mammary glands, Harderian glands, and clitoral glands and preputial glands.
In humans, salivary gland myoepitheliomas are classified based on morphology (plasmacytoid, spindle, stellate, clear or epithelioid) of the neoplastic cells into spindle cell myoepithelioma, clear cell myoepithelioma, etc. Due to the histologic heterogeneity, no single immunostain is diagnostic. For human salivary myoepitheliomas, a panel of markers consisting of AE1/AE3 (PAN-K), S-100, P63, GFAP, calponin and vimentin is commonly used for diagnosis.(4) A panel of markers, such as vimentin and cytokeratin (especially k5 and k14), may be used for immunohistochemical diagnosis of these tumors in mice. Also, PTAH staining will aid in confirmation of the presence of intracytoplasmic fibrils within the neoplastic epithelioid cells. Ultrastructurally, these fibrils are composed of abundant microfilaments in parallel orientation with periodic focal densities, characteristic of smooth muscle fibrils, and are arranged in dense parallel bundles around the nucleus.(3) These tumors are usually negative for smooth muscle actin and desmin.
Acknowledgements: We appreciate the help of Drs. Terry Blankenship-Paris, Mark Hoenerhoff, and Steven Kleeberger at NIEHS, RTP, NC for graciously sharing the case material.
JPC Diagnosis:
Conference Comment:
Participants discussed the differential diagnosis, which included poorly differentiated carcinoma/adenocarcinoma, carcinosarcoma, and sarcoma arising or metastatic to glandular organs, such as the salivary, mammary, lacrimal, and Harderian glands. All participants interpreted the tumor as arising from the salivary gland, and thus considered a neoplasm of other regional glandular tissues less likely, including mammary, lacrimal, or Harderian origin. Based on the absence of desmoplasia and glandular or squamous differentiation, participants considered a tumor of epithelial origin less likely; the glandular or ductular profiles occasionally found in the neoplasm were interpreted as preexisting salivary structures entrapped by neoplastic cells. Participants did observe the large pseudocystic structures in the neoplasm described by the contributor, which were not interpreted as evidence of glandular differentiation.
Finally, participants discussed the difficulty in the histologic differentiation of salivary myoepithelioma from polyoma virus-induced salivary neoplasms. Polyoma virus inoculated into neonatal mice of susceptible strains induces tumors in multiple organs, in particular the parotid salivary gland.(2) Histologically, polyoma virus-induced salivary neoplasia most commonly occurs as mixed mesenchymal and epithelioid populations, although neoplasms composed of pure mesenchymal or epithelioid populations may be observed.(2) In contrast to polyoma virus-induced salivary tumors, myoepitheliomas typically are not infiltrated by lymphocytes and plasma cells.(1)
The use of tissue from multiple animals in this case contributed to slide variation, with some slides having one type of salivary gland and others having more than one type present.
References:
2. Botts S, Jokinen M, Gaillard ET, Elwell MR, Mann PC: Salivary, Harderian, and lacrimal gland glands. In: Maronpot RR, ed. Pathology of the Mouse. Vienna, IL: Cache Valley Press; 1999:56-60.
3. Burger GT, Frith CH, Townsend JW. Myoepithelioma, Salivary glands, mouse. In: Jones TC, Popp JA, Mohr U, eds. Digestive system. Monographs on Pathology of Laboratory Animals. Berlin, Germany: Springer-Verlag; 1997:231-235
4. Hunt JL, Barnes L. Immunohistology of head and neck neoplasms. In: Dabbs D, ed. Diagnostic Immunohistochemistry. 2nd ed. Philadelphia, PA: Churchill Livingstone (Elsevier Inc.); 2006:245-247.
5. Sundberg JP, Hanson CA, Roop DR, Brown KS, Bedigian HG. Myoepitheliomas in inbred laboratory mice. Vet Pathol. 1991;28:313-323.