Signalment:
Gross Description:
Histopathologic Description:
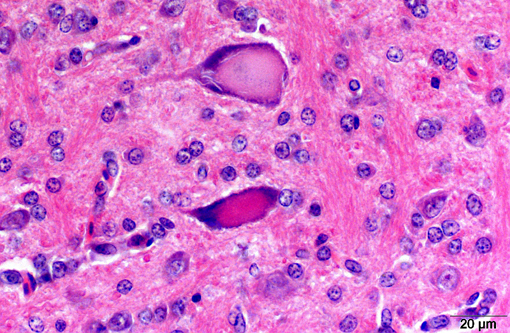
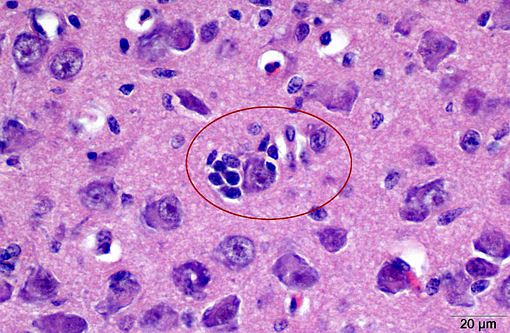
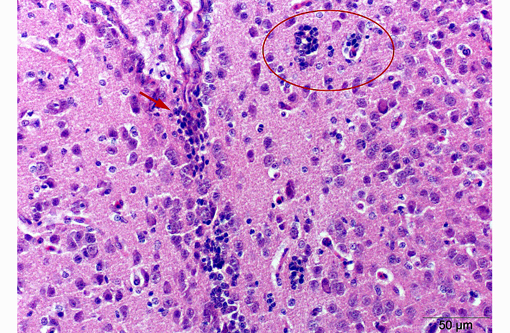
With some slide variation, few neuronal cell bodies are surrounded by low numbers of astrocytes (satellitosis). Occasionally, macrophages phagocytosing cellular and karryorhectic debris (neuronophagia) as well as discrete nodules composed of low numbers of glial cells and fewer lymphocytes replacing neurons (glial nodules) can be observed. Scattered minimal to mild predominantly perivascular lymphocytic and histiocytic infiltrates (lymphohistiocytic cuffing) are present.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Clinically, outbreaks occur in young birds, less than six (typically 1-3) weeks of age. The animals develop ataxia with possible progression to paralysis or tremor of the head and neck (i.e., epidemic tremor). In general, the severity of the disease depends on the age and immunological status of the bird. Whereas clinical signs might be present at the time of hatching, they are usually evident between 1-2 weeks of age. There seems to be a marked resistance if exposure is after 2-3 weeks of age. Adult birds show a drop in egg production for no more than two weeks.(1,2)
Gross lesions are commonly absent, especially in adult chickens. Whitish areas in the ventriculus muscle due to massive lymphocytic infiltration may occur.(1,2) Histologically, lesions may be lacking in the peracute stage. Typical lesions of acute AE include central chromatolysis of neurons in the brainstem (medulla oblongata) and of Purkinje cells. The neuronal nuclei isthmi, ruber, reticularis, rotundus and cerebellaris as well as the ventral horns of the spinal cord are primary targets. A disseminated lymphoplasmahistiocytic encephalomyelitis with ganglionitis of the dorsal root ganglia can be detected in more subacute to chronic cases. In addition, nodular microgliosis, predominantly in the cerebellar molecular layer, may be observed. Multifocal microgliosis of the Purkinje cell layer, with triangular or flame-like extensions into the molecular layer are also suggestive of AE. Additionally, aggregates of lymphocytes appear within the muscular wall of the ventriculus, the pancreas and myocardium.(1,2)
Differential diagnoses include Mareks disease virus, which causes lymphoid infiltrates in the peripheral nerves and lymphomatosis of the viscera (not seen in AE), Newcastle disease virus, with peripheral chromatolysis rather than the central chromatolysis of AE, and Avian influenza virus.(1,2)
JPC Diagnosis:
Conference Comment:
The Picornaviridae family is composed of eight genera: Aphthovirus, Enterovirus, Teschovirus, Cardiovirus, Erbovirus, Kobuvirus, Hepatovirus and Parechovirus. Picornaviruses are non-enveloped, icosahedral, single stranded, RNA visions.(4) Diseases of veterinary importance caused by picornaviruses include foot-and-mouth disease (Aphthovirus), encephalomyocarditis virus and Theilers mouse encephalomyelitis virus (Cardiovirus), swine vesicular disease (Enterovirus), porcine teschovirus 1 (Teschovirus) and avian encephalomyelitis virus. Originally classified in the genus Enterovirus and later reclassified into the genus Hepatovirus, AEV has recently been considered for classification into a new genus within the Picornaviridae family, Tremovirus.(4)
The striking feature of this case is the paucity of microscopic lesions, consistent with the peracute disease course described in this case. Conference participants debated the presence of Purkinje cell degeneration and central chromatolysis, however a consensus was not reached. The hypercellularity of the submitted specimen also confounded uniform diagnosis in this case, as development of the brain is still ongoing in a 1-day-old chick, and differentiation of maturing, migrating, and developing cells of neural and glial origin from those associated with viral infection.
References:
2. Avian Histopathology. 3rd ed. Madison, WI: American Association of Avian Pathologists; 2008:265-266.
3. Jones EE. An encephalomyelitis in the chicken. Science. 1932;76:331-332.
4. MacLachlan NJ, Dubovi EJ, eds. Fenners Veterinary Virology. 4th ed. London, UK: Academic Press; 2011:425-441.
5. Tannock GA, Shafren DR. Avian encephalomyelitis: a review. Avian pathology: Journal of the WVPA. 1994;23:603-620.
6. Welchman DdeB, Cox WJ, Gough RE, et al. Avian encephalomyelitis virus in reared pheasants: a case study. Avian Pathol. 2009;38(3):251-256.