Signalment:
Gross Description:
Histopathologic Description:
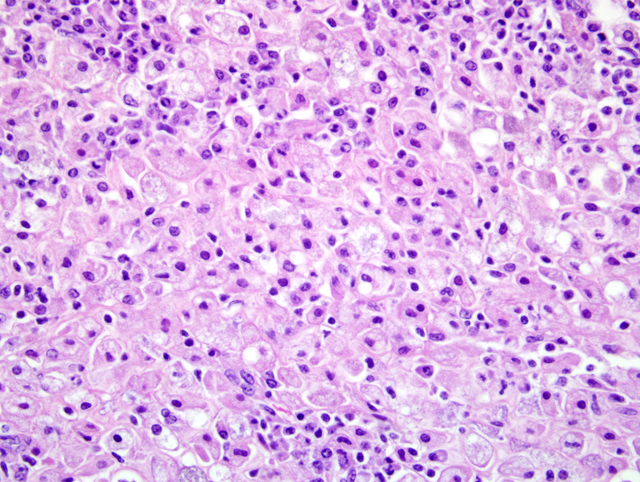
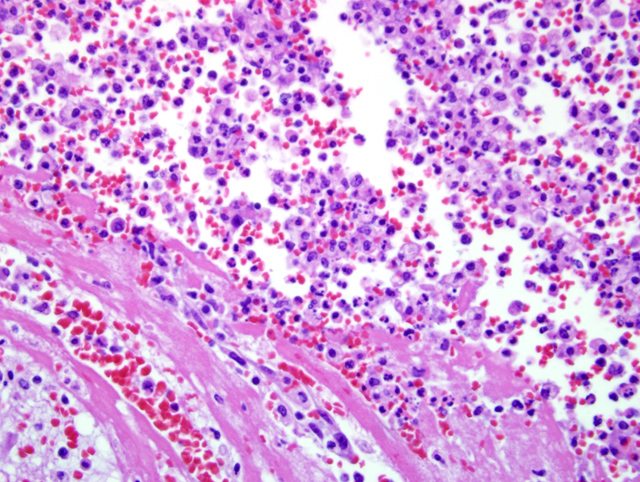
Select mesenteric lymph nodes are multifocally expanded and disrupted by large numbers of macrophages that contain abundant cytoplasm, cellular and karyorrhectic debris, edema, and hemorrhage (Fig. 1-2).Â
The mucosa of the small intestine is multifocally eroded. Occasionally cellular debris may be found in the lumen and in the submucosa. Lymphocytes and plasma cells mildly expand the submucosa. There are moderate numbers of bacilli multifocally within the lumen, on the surface of erosive areas, and invading the mucosa and submucosa.
Morphologic Diagnosis:
1. Mesentery and ileum: Peritonitis and serositis, histiocytic, chronic, multifocal, marked, with fibrosis, necrosis, and rare hemorrhage, African green monkey (Chlorocebus aethiops)
2. Lymph node, mesenteric: Lymphadenitis, histiocytic, subacute, multifocal, moderate, with necrosis and hemorrhage
3. Small Intestine: Enteritis, erosive, multifocal, mild to moderate, with bacilli
Lab Results:
Condition:
Contributor Comment:
Klebsiella pneumoniae is a gram-negative, aerobic, nonmotile bacillus and is a common cause of a wide range of infections in humans and animals.(6) Our differential diagnosis for Gram-negative pathogens in nonhuman primates included, but were not limited to, Yersinia enterocolitica (forms large colonies in tissues, which were not present in our cases), Shigella sp. (S. dysenteriae, S. Flexneri, S. boydii, S. sonnei), Campylobacter sp. (C. jejuni, C. coli), Salmonella sp. (S. typhimurium, S. Dublin, S. enteriditis, S. Stanley). In Old and New World monkeys, infection with K. pneumoniae causes pneumonia, meningitis, peritonitis, cystitis, and septicemia.(9) K. pneumoniae also constitutes normal fecal and oral flora in many nonhuman primates. In the past two decades, a new type of invasive K. pneumoniae disease has emerged in humans in Taiwan and other Asian countries, and more recently from non-Asian countries, including the USA.(3,4,5,7) Fatal human infections with invasive strains of K. pneumoniae involve pulmonary emboli or abscess, meningitis, endophthalmitis, osteomyelitis, or brain abscess. Recently, a highly invasive K. pneumoniae causing primary liver abscesses in humans has also been reported.(3) These invasive, abscess-forming strains of K. pneumoniae are associated with the so-called hypermucoviscosity (HMV) phenotype, a bacterial colony trait identified by a positive string test.(4,5,6) The HMV phenotype is seen in K. pneumoniae expressing either the capsular serotypes K1 or K2. K1 serotypes of K. pneumoniae have two potentially important genes, rmpA, a transcriptional activator of colanic acid biosynthesis, (10) and magA, which encodes a 43-kD outer membrane protein. Five K2 serotypes of K. pneumoniae also have rmpA but do not have magA. Capsular serotypes K1 and K2 are reported to play an important role in the invasive ability of HMV K. pneumoniae.(3,10) The role of rmpA and magA in the pathogenesis of invasive K. pneumoniae, however, seems less certain. K. pneumoniae expressing the HMV phenotype has not been reported to cause natural disease in nonhuman primates, nor in other animal species.Â
The means by which the causative K. pneumonia may have spread or caused disease in individual monkeys in our colony is unknown. The only significant epidemiologic factor we identified was that affected monkeys were maintained in the same room or had contact with a monkey housed in that room. African green monkeys may be quite susceptible to invasive K. pneumoniae infection. Therefore, veterinarians, laboratory workers, and research pathologists should be aware of this pathogen as a cause of abdominal masses and multisystemic abscessation in the AGM. In addition, the AGM may provide another useful animal model to understand the pathogenesis of this emerging human pathogen.
JPC Diagnosis:
1. Ileocecocolic junction: Serositis and peritonitis, granulomatous, multifocal to coalescing, severe with marked fibrosis
2. Lymph node, mesenteric: Lymphadenitis, pyogranulomatous, diffuse, severe
Conference Comment:
In domestic animals, Klesiella pneumonia is the cause of numerous maladies. In foals, K. pneumonia is a common cause of neonatal septicemia and pneumonia.(1,2) K. pneumonia has also been commonly implicated in equine abortions.(8)
References:
2. Caswell JL, Williams KJ: In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., pp. 632. Elsevier, Philadelphia, Pennsylvania, 2007
3. Chuang YP, Fang CT, Lai SY, Chang SC, Wang JT: Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 193:645654, 2006
4. Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT: A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med 199:697705, 2004
5. Fang FC, Sandler N, Libby SJ: Liver abscess caused by magA+ Klebsiella pneumoniae in North America. J Clin Microbiol 43:991992, 2005
6. Kawai T: Hypermucoviscosity: an extremely sticky phenotype of Klebsiella pneumoniae associated with emerging destructive tissue abscess syndrome. Clin Infect Dis 42:13591361, 2006
7. Rahimian J and JT Wang: The Emerging Role of Klebsiellae in Liver Abscess. In: Emerging Infections, eds. Scheld WM, DC Hooper, and JM Hughes, pp. 199-211. ASM Press, Washington, DC, 2007
8. Schlafer DH, Miller RB: Female genital system. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, vol 3 ed. Maxie MG, pp. 507. Elsevier Limited, Philadelphia, PA, 2007
9. Twenhafel NA, Whitehouse CA, Stevens EL, Hottel HE, Foster CD, Gamble S, Abbott S, Janda JM, Kreiselmeier N, Steele KE: Multisystemic abscesses in African green monkeys (Chlorocebus aethiops) with invasive Klebsiella pneumoniae-identification of the hypermucoviscosity phenotype. Vet Pathol 45:2, 2008
10. Yu WL, Ko WC, Cheng KC, Lee HC, Ke DS, Lee CC, Fung CP, Chuang YC: Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis 42:13511358, 2006