Signalment:
Experimental History: This monkey received a single 7.55 Gy dose of whole-body irradiation at 3 years of age.
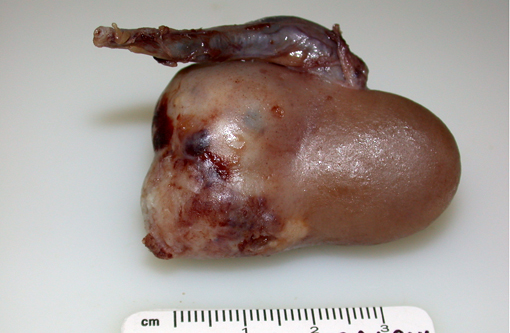
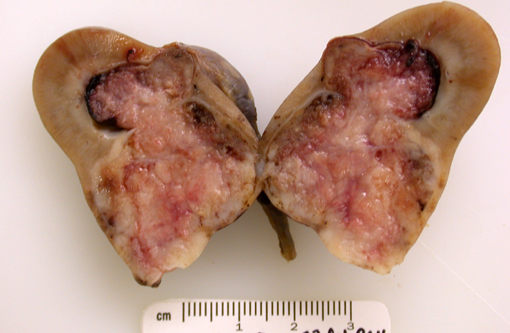
Gross Description:
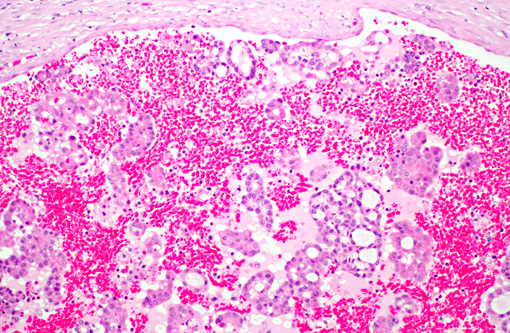
Histopathologic Description:
Morphologic Diagnosis:
1. Papillary carcinoma, kidney and ureter with vascular invasion
2. Glomerulonephropathy, diffuse, subacute to chronic, mild, with interstitial fibrosis, glomerular atrophy, tubular loss and cyst formation
Lab Results:
Blood Chemistry:
BUN 13 mg/dL (13-27 mg/dL)
Creatinine 1.5 mg/dL (0.8-1.5 mg/dL)
Urinalysis:
Specific Gravity 1.007
Blood 80 cells/mL
Protein: 2+ (100 mg/dL)
Bacteria: none
WBC: 6-10/HPF
RBC: 30-50/HPF
Condition:
Contributor Comment:
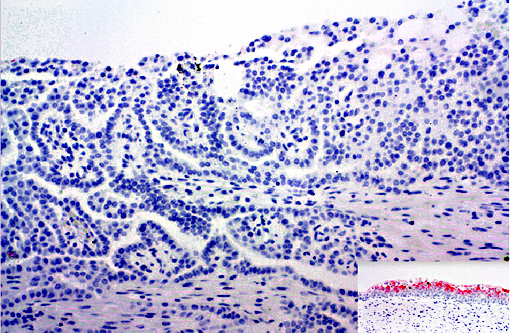
Abnormalities were not detected in the urinary bladder during the CT scan or visible during the nephrectomy surgery in this animal. This combined with negative uroplakin III staining makes an urothelial carcinoma less likely. However, urothelial carcinomas in humans may not stain for uroplakin III, particularly those with invasive or metastatic behaviors(15). In humans, renal carcinomas are further characterized based on morphology(14, 15).
Although mild, the nephropathy is likely secondary to irradiation. Irradiation-induced nephropathy is a significant long-term complication in rhesus macaques and humans6, 16. The changes in rhesus macaques, 6-8 years after a single dose of irradiation (7.2-8.5 Gy) include glomerulopathy, ectatic capillaries, glomerulosclerosis, and periglomerular fibrosis(16). About 25% of human patients receiving comparable doses of radiation (7-7.5 Gy) develop renal dysfunction(6).
JPC Diagnosis:
1. Kidney: Renal papillary carcinoma.
2. Kidney: Glomerulonephropathy characterized by interstitial fibrosis, tubular degeneration and regeneration, proteinosis, and lymphoplasmacytic interstitial nephritis.
Conference Comment:
Some renal adenocarcinomas in dogs have elevated expression of COX-2, suggesting COX-2 mediated prostaglandins may play a role in the modulation of neoplastic cell growth(7). A notable paraneoplastic syndrome and common clinical pathology finding is secondary absolute polycythemia due to secretion of erythropoietin or erythropoietin-like peptide by the tumor(12).
Grossly, renal adenocarcinomas appear as large (>2 cm), spherical to ovoid, well-demarcated masses that are usually unilateral and occupy one pole of the kidney. They usually arise in the cortex and compress adjacent renal parenchyma, and may occupy 80% or more of the kidney. They often present as light yellow to gray lobulated masses with areas of necrosis and hemorrhage. Common sites of metastasis are the lungs, regional lymph nodes, liver, and occasionally the skin. Invasion into the renal vein and posterior vena cava may can occur. Multiple and bilateral renal neoplasms, without evidence of metastasis to other organs, are considered to be of multi centric origin(9, 10, 12).
Histologically, these neoplasms are often described by the predominant histologic type and further sub-classified by the predominant cytologic type, although there is no prognostic significance to histologic or cytologic type. Histologic types include papillary, tubular, and solid, and a mixture of all three patterns may be present in any one tumor. The tubular variant is most common in domestic animals, and often, the solid variant is usually poorly differentiated. There are several cytologic types, such as chromophobic, eosinophilic, and clear cell (with vacuolated cytoplasm) (9, 10, 12). The clear cell variant is more often seen in laboratory animals and they tend to be solid rather than tubular. Electron micrographs, may demonstrate abundant monoparticulate glycogen often within phago-lysosomes and few mitochondria or endoplasmic reticulum(10). Uromodulin (Tamm-Horsfall glycoprotein), a unique protein produced by the kidney, is useful as an immunohistochemical marker(9).
Differential diagnoses include oncocytoma, nephroblastoma and transitional cell carcinoma(9, 10, 12).
References:
2. Broerse JJ, Bartstra RW, van Bekkum DW, van der Hage MH, Zurcher C, van Zwieten MJ, Hollander CF. The carcinogenic risk of high dose total body irradiation in non-human primates. Radiotherapy and Oncology 2000; 54:247-253.
3. Broerse JJ, van Bekkum DW, Zurcher C. Radiation carcinogenesis in experimental animals. Experientia 1989; 45: 60-69.
4. Epstein, JI. The Lower Urinary Tract and Male Genital System. In: Robbins and Cotran Pathologic Basis of Diseases. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010: 971-1004.
5. Hollander CF, Zurcher C, Broerse JJ. Tumorigenesis in High-Dose Total Body Irradiated Rhesus Monkeys A Life Span Study. Toxicologic Pathol 2003; 31(2): 209-213.
6. Kal HB, van Kempen-Harteveld ML. Renal Dysfunction after Total Body Irradiation: Dose-effect Relationship. Int J Radiation Oncology Biol Phys 2006; 64(4): 1228-1232.
7. Khan KNM, Stanfield KM, Trajkovic D, Knapp DW: Expression of cyclooxygenase-2 in canine renal cell carcinomas. Vet Pathol 38:116-119, 2001
8. Lingaas F, et al. A mutation in the canine BHD gene is associated with hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis in the German shepherd dog. Hum Mol Genet. 2003; 12(23):3043-53.
9. Maxie MG, Newman SJ: Urinary system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed., Maxie MG, 5th ed., Vol 2 pp. 498-501. Elsevier Limited, New York, NY, 2007
10. Meuten D: Tumors of the urinary system. In: Tumors in Domestic Animals, ed. Meuten D, 4th ed., pp. 509-518. Iowa State Press, Ames, IA, 2002
11. Meuton, DJ. Tumors of the urinary system. In: Meuten DJ, ed. Tumors of Domestic Animals 4th ed. Ames, IA: Iowa State Press; 2002:380-392.
12. Newman SJ, Confer AW, Panciera RJ: Urinary system. In: Pathologic Basis of Veterinary Disease, ed., McGavin MD, Zachary JF, 4th ed., pp. 640-1. Mosby, St. Louis, MO, 2007
13. Suit H, Goldberg S, Niemierko A, Ancukiewicz M, Hall E, Goitein M, Wong W, Paganetti H. Secondary Carcinogenesis in Patients Treated with Radiation: A Review of Data on Radiation-Induced Cancers in Human, Non-human Primate, Canine and Rodent Subjects. Radiat Res 2007; 167: 12-42.
14.Tickoo SK, Reuter VE. Differential Diagnosis of Renal Tumors With Papillary Architecture. Adv Anat Pathol 2011; 18:120-132.
15.Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med2011; 135:92-109.
16. van Kleff EM, Zurcher C, Oussoren YG, Te Poele JAM, van der Valk MA, Niemer-Tucker MMB, van der Hage MH, Broerse JJ, Robbins MEC, Johnston DA, Stewart FA. Long-term effects of total-body irradiation on the kidney of Rhesus monkeys. Int J Radiat Biol 2000; 76(5): 641-648.