WSC 22-23
Conference 7
CASE I:
Signalment:
A 17-day-old, male, Japanese Black, bovine
History:
A Japanese black male calf was born at full-term. The calf weighted 30 kg and received colostrum. The calf presented with fever (40 ?), cough, nasal discharge, poor suckling, wobbler, and bilateral ocular cloudiness on day 4 of life, after which he developed astasia. On day 5 of life, the calf became recumbent and presented with dizziness and nystagmus. The calf was treated with medications such as antibiotics, non-steroid anti-inflammatory drugs, and vitamin B1, but he died on day 17 of life.
Gross Pathology:
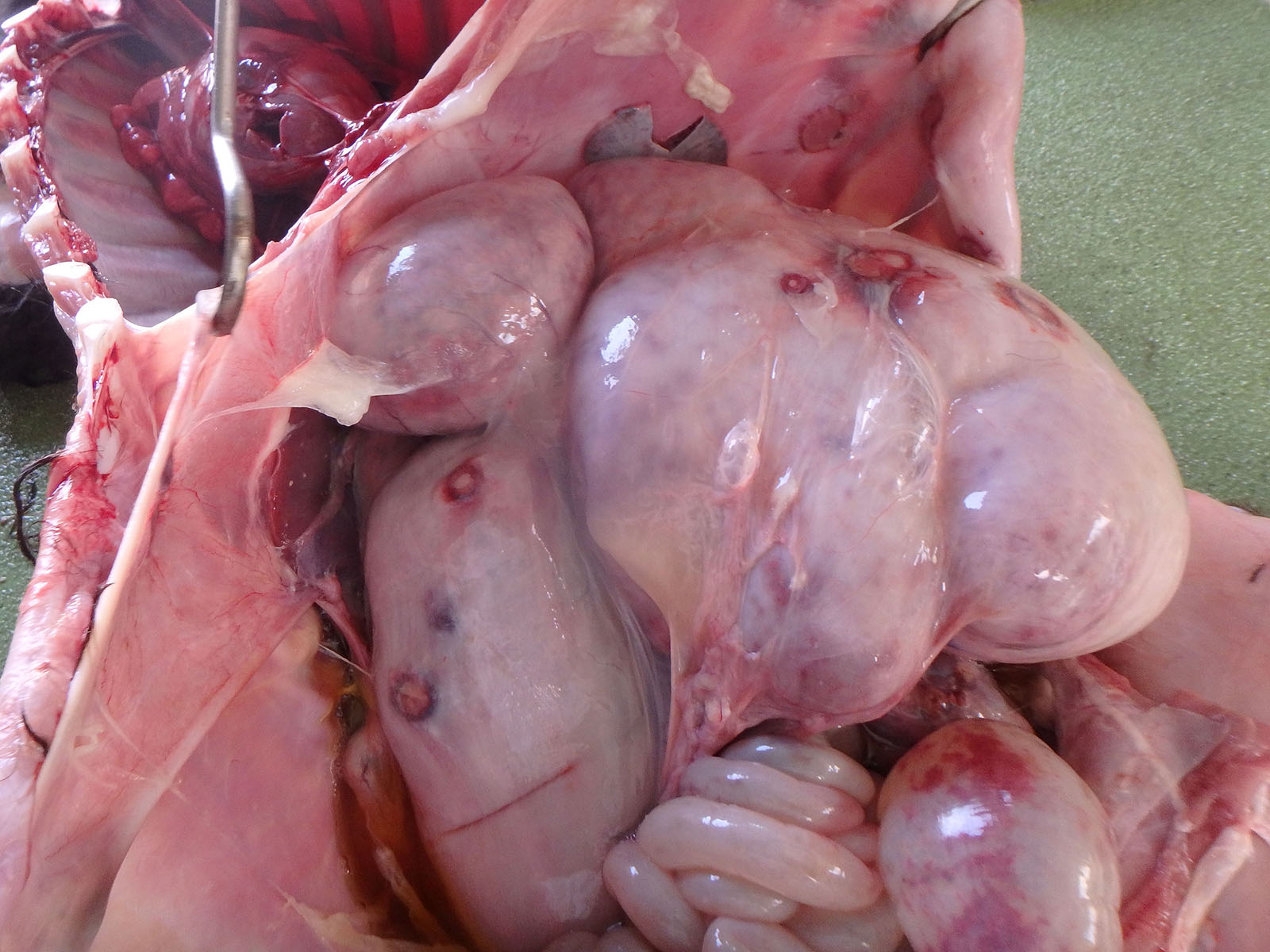
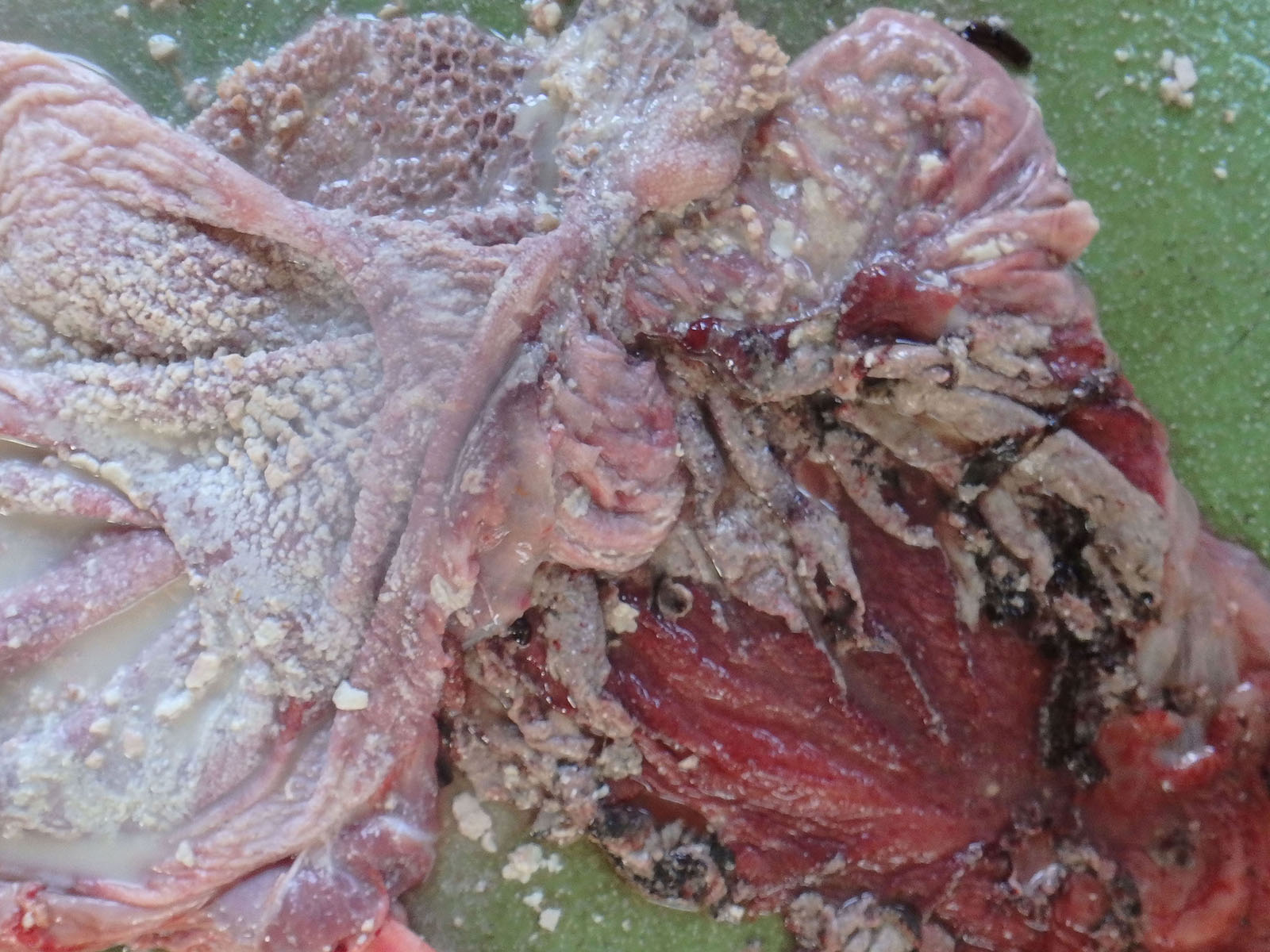
Multiple white nodules with dark red edges and a diameter of?1 - 3 cm were found scattered in the peritoneum, including the spleen, stomach, and diaphragm. Many white nodules were also observed in the liver serosa and parenchyma. The surface of the forestomach mucosa was covered with a gray-yellowish caseous substance. Multiple erosions and ulcers were observed in the forestomach and the abomasum. The abomasum mucosa was extensively dark red. The calf had an increased volume of the cerebrospinal fluid, which was muddy; thymic hypoplasia; and persistent urachus.
Laboratory Results:
Bacteriological examination: Yeast-like fungi were isolated from the samples collected from the liver, kidney, lung, brain, and cerebrospinal fluid, which were identified as Candida albicans (99.12%) on sequence analysis of the ITS region.
Virological examination: Bovine adenovirus (BAdV) type 4 was detected on the PCR for BAdV and sequence analysis of DNA extracted from paraffin-embedded sections of the liver, reticulum, and ileocecal colon.
Microscopic Description:
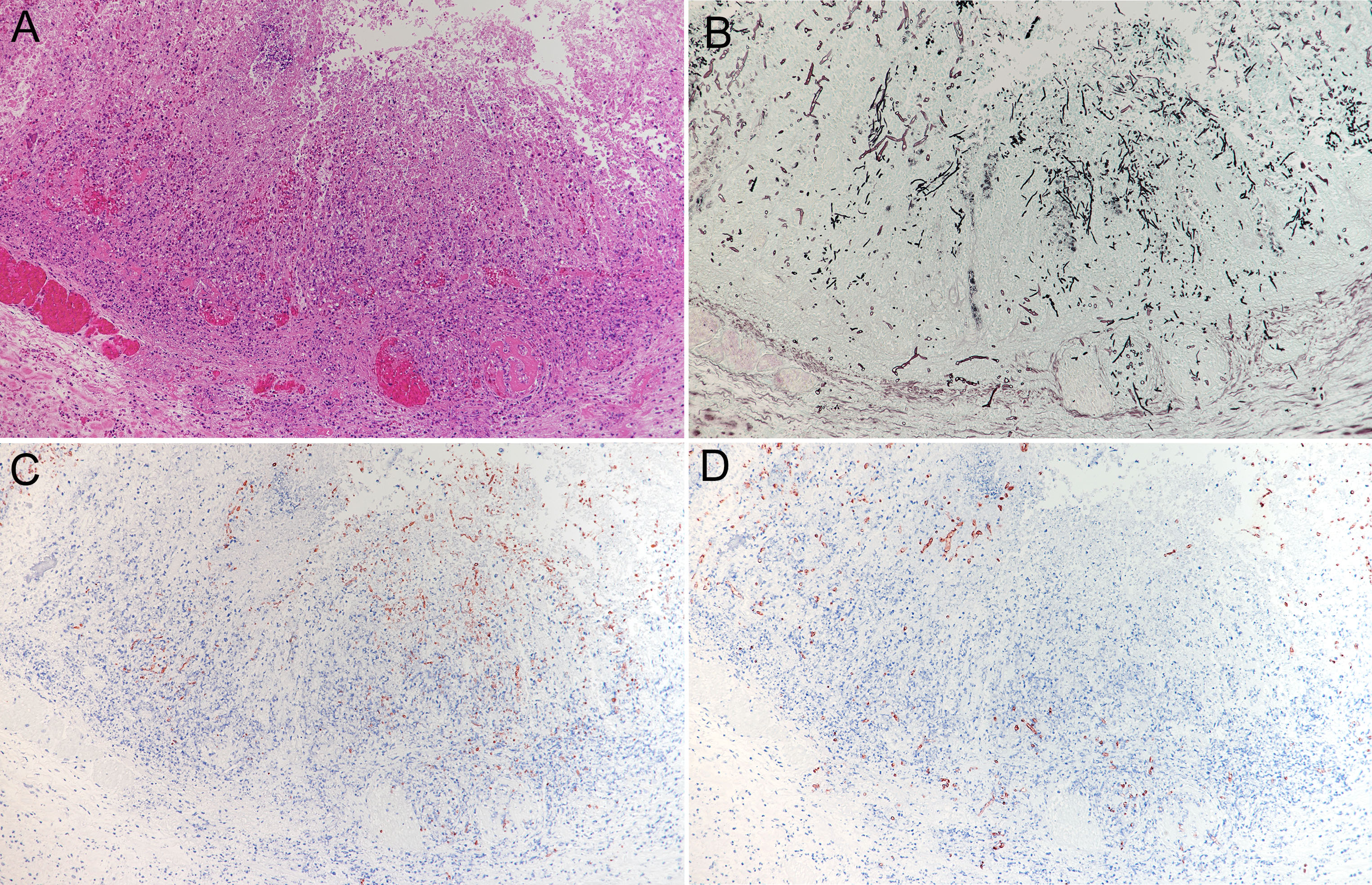
Multiple extensive necroses were observed from the mucosal epithelium to the submucosal tissue of the abomasum. In the necrotic area, typical structures were replaced by hemorrhage, acidophilic cell debris, and mostly necrotic infiltrated neutrophils. Numerous thrombi were observed in small-to-medium blood vessels from the lamina propria to the submucosa. Numerous fungal hyphae were found in the necrotic lesions. Presence of fungi was also found in thrombi and on the walls of blood vessels. The submucosa was highly edematous, with numerous neutrophil infiltrations, mild macrophage infiltrations, and presence of fibrin. Grocott’s staining and PAS reaction revealed the presence of multiple species of fungi in the lesion. On the mucosal surface, a large number of yeasts and pseudohyphae were found mixed with inflammatory cells and cell debris. A large number of hyphae were found from the necrotic area to the submucosa. They were 5-8 µm wide, non-parallel, thin walled, and irregularly branched and had no septum. Such hyphae had also infiltrated the thrombus and walls of blood vessels. In some areas, these hyphae were mixed with pseudohyphae, which invaded the blood vessels. In the submucosa adjacent to these areas, a large number of hyphae were focally observed in the blood vessels, blood vessel walls, and surrounding tissues. They had slightly thicker walls; were 4-5 µm wide, parallel, and sharply branched; and contained septum. Immunohistochemically, these fungi reacted positively to anti-Candida (Biogenesis, UK), anti-Rhizomucor (Clone Mab-WSSA-RA-1?DAKO?USA), and anti-Aspergillus (Clone Mab-WF-AF-1?DAKO?USA) antibodies, respectively.
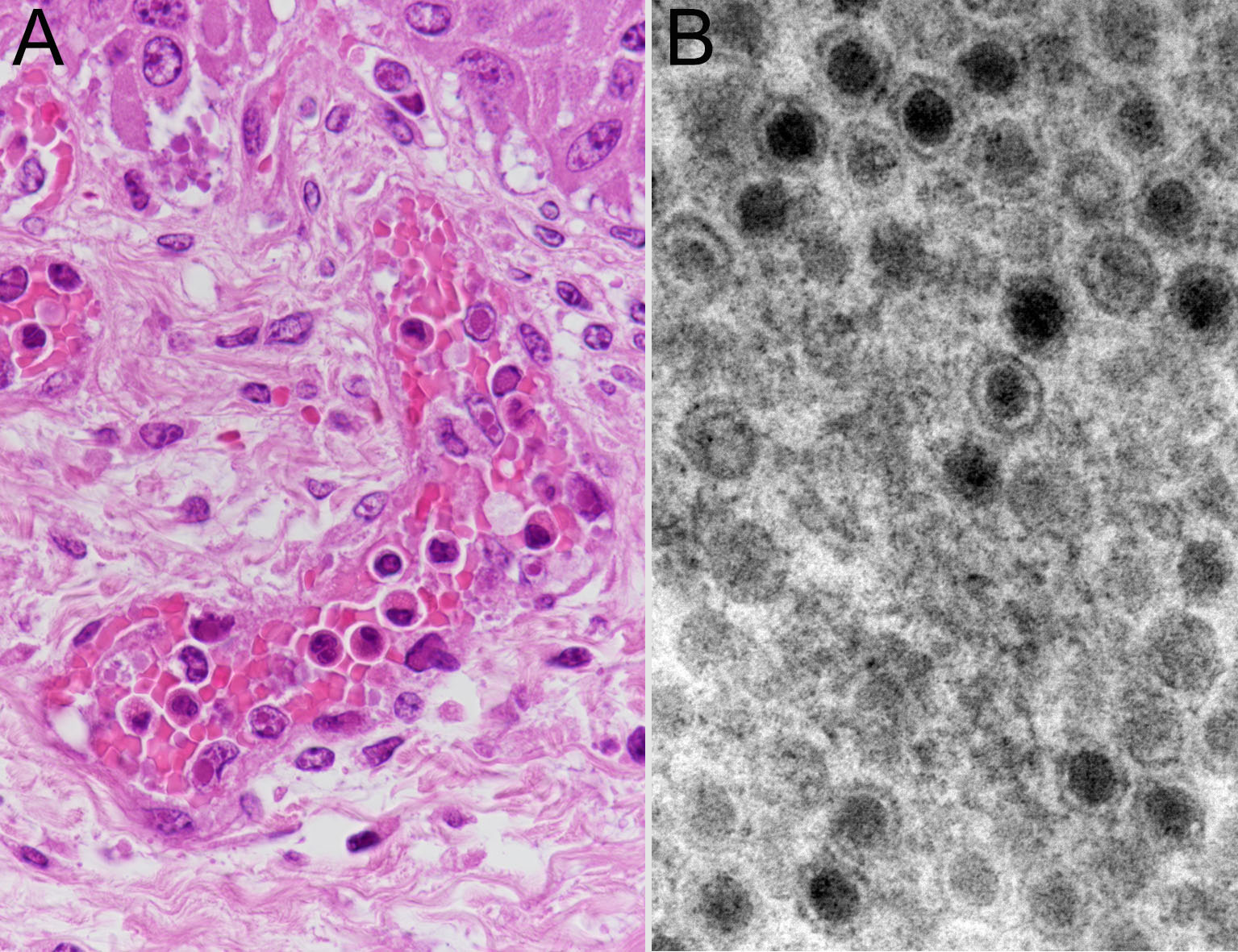
Furthermore, amphoteric-to-basophilic Cowdry A-type or full-type intranuclear inclusion bodies were observed in endothelial cells of most blood vessels. Electron microscopy of vascular endothelial cells revealed adenovirus-like particles with a diameter of approximately 80 nm, consistent with intranuclear inclusion bodies.
In the forestomach, severe hyperkeratosis or parakeratosis, mild-to-moderate erosion of the epithelium, and intoraepithelial accumulation of neutrophils (microabscesses) were observed. A large number of anti-Candida antibody-positive yeasts and pseudohyphae were observed in these lesions. In addition, numerous thrombi and extensive necrosis of the surrounding tissues were also observed in the rumen submucosa. In the necrotic tissue and blood vessels, anti-Aspergillus antibody-positive hyphae were observed. Anti-Aspergillus antibody-positive hyphae were also observed in the serosa. In other regions, a large number of anti-Rhizomucor antibody-positive hyphae in the vascular wall and necrotic foci of the lamina propria were observed. There were numerous viral intranuclear inclusion bodies in the vascular endothelium of the forestomach. In the ileum, swelling of vascular endothelial cells, presence of thrombi with numerous intranuclear inclusions scattered from the lamina propria to the submucosa, and necrosis with hemorrhage were widely observed.
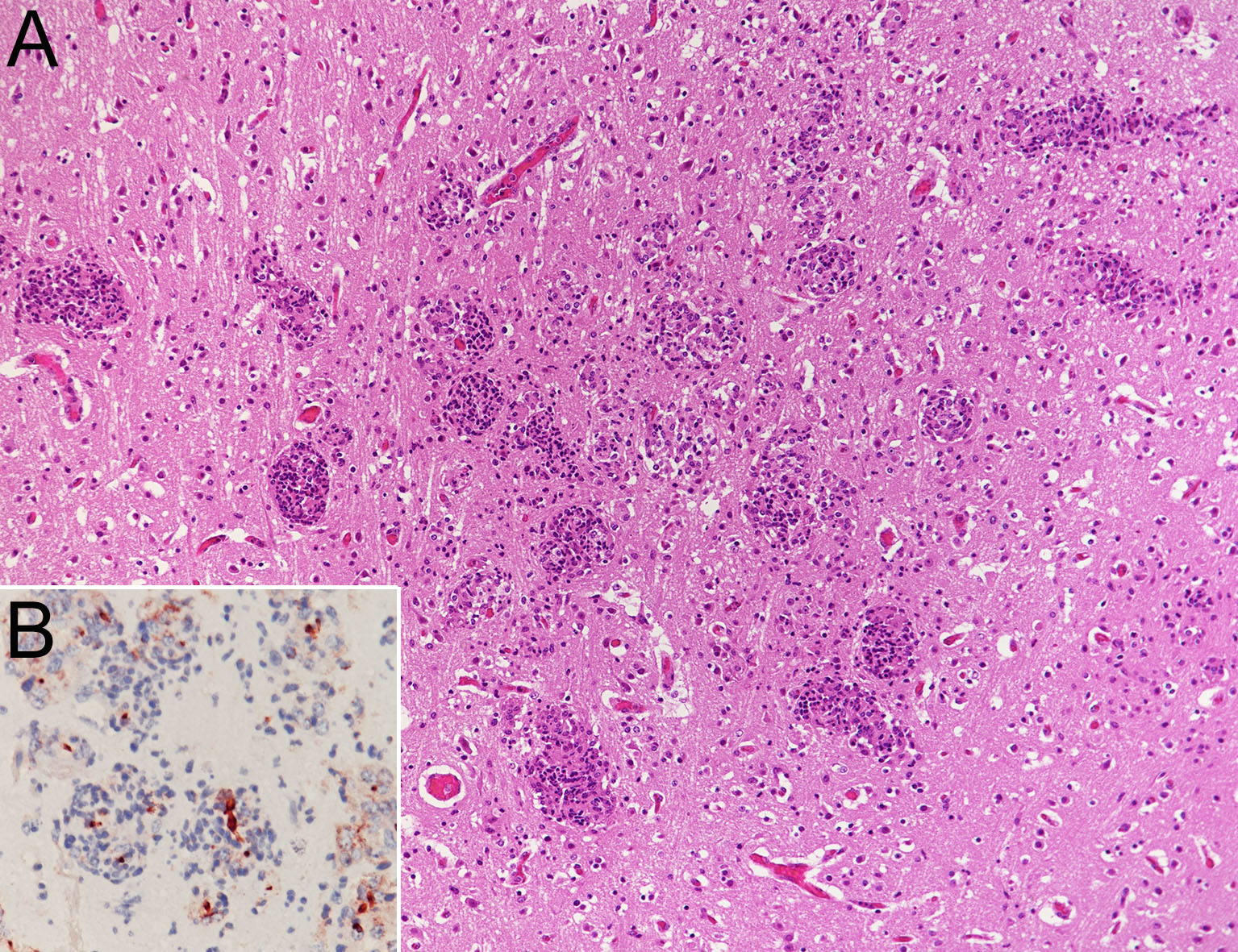
Many focal necroses or granulomas with mild hemorrhage were scattered in the brain and the spinal cord. A large number of anti-Candida antibody-positive pseudohyphae were observed in these lesions. Mild mononuclear cell infiltration was observed in the meninges, and one mycotic granulomatosis was observed in the choroid plexus. Intranuclear inclusion bodies were rarely observed in vascular endothelial cells.
Focal extensive necrosis with thrombosis was observed in the liver. Many hyphae that reacted against the anti-Aspergillus antibody were observed in thrombi and vascular wall. Furthermore, numerous focal necroses with anti-Candida antibody-positive pseudohyphae were randomly scattered. In addition, a number of intranuclear inclusion bodies were found in the endothelium or hepatocytes. A few anti-Candida antibody-positive pseudohyphae were observed around small vessels in the kidney, heart, and lung.
In the thymus, there was severe depletion of lymphocytes in both the cortex and medulla.
Contributor’s Morphologic Diagnoses:
Abomasum: abomasitis, necrotizing, severe with vasculitis, vascular thrombosis, numerous multiple species of fungi, and viral intranuclear inclusion bodies
Etiology: Candida albicans, Aspergillus spp., Mucoraceae, and BAdV type 4
Contributor’s Comment:
In this case, fungal lesions caused by C. albicans were systemically disseminated in many organs. Co-infection with Aspergillus spp. and Mucoraceae was observed in fungal lesions of the gastrointestinal tract and liver. Systemic BAdV type 4 infection was also observed.
Deep-seated mycosis is a fungal infection that forms lesions in the internal organs or subcutaneous tissue. In cattle, mastitis, endometritis, ulcerative gastroenteritis, pneumonia, and abortion have been reported.16 Deep-seated mycosis occurs in animals with weakened immunity, and the main causative fungi are Candida spp., Aspergillus spp., and Mucoraceae,2,4,7,15,16 either alone1,5,6,12 or in combination2,3,14. In cattle with deep-seated mycosis, co-infection with two types of fungi3,13,14 and mixed infection with fungi and protozoa have been reported.13 Many cases of systemically disseminated mycosis in cattle caused by Mucoraceae, resulting in infection of central nervous system tissues, have been reported.1,4-6,12,13 In contrast, a few cases of central nervous system lesions caused by Aspergillus spp.,3 without the presence of Candida spp. have been reported. In the present case, co-infection with three species of fungi was observed. C. albicans was identified as the causative fungi for lesions systemically disseminated to multiple organs, especially meningoencephalomyelitis.
A large number of Aspergillus spp. hyphae were observed in the liver, rumen, and abomasum, and a large number of Mucoraceae hyphae were observed in the rumen and abomasum. Both fungi were associated with thrombosis, vasculitis, and extensive necrotic lesions. Numerous yeasts and pseudohyphae of C. albicans were observed in the hyperkeratotic and parakeratotic mucosal epithelium and superficial lamina propria lesions of the forestomach, and they were not mixed with other fungi. However, in the abomasum, pseudohyphae of C. albicans were observed in the necrotic foci mixed with Mucoraceae hyphae along with invasion into the deep mucosa or blood vessels. These findings suggest that C. albicans was disseminated systemically through necrosis and vascular lesions caused by Mucoraceae in the abomasum.
On the other hand, viral intranuclear inclusion bodies were observed in vascular endothelial cells throughout the body, such as in the gastrointestinal tract, liver, and brain. Electron microscopy and sequence analysis of DNA extracted from FFPE samples showed that the inclusion bodies were BAdV type 4. BAdV disease is caused by BAdV belonging to the Mastadenovirus genus or the Atadenovirus genus of the Adenoviridae family.18 It is mainly characterized by respiratory and digestive diseases. BAdVs are commonly isolated from the feces of healthy cattle,11 which are rarely onset by BAdV alone. There are 10 serotypes of BAdV, and BAdV type 7 is highly pathogenic, 18 causing severe hemorrhagic diarrhea and calf frailty syndrome. Only a few cases of BAdV disease have been reported in Japan.8,9 BAdV type 4 was identified only in one of these case via bovine testis cell culture.8 Gastric erosion, ulcers, and hemorrhagic colitis have been reported in previous cases of BAdV infection.17,18 In the present case, mucosal necrosis without fungal infection was observed in the ileum. It was suggested that BAdV type 4 injured blood vessels and induced gastrointestinal lesions. Furthermore, it was considered that C. albicans entered the bloodstream and invaded the parenchyma of the brain and liver through the vascular walls systemically damaged by the virus, causing inflammation or necrosis.
In addition, thymic hypoplasia, which was observed in the present case, may have contributed to these systemic infections.
Many cases of zygomycosis are angioinvasive and disseminated systemically.1,5,6 However, no systemic dissemination of Mucoraceae was observed in the present case, probably because the calf developed fatal meningoencephalomyelitis due to C. albicans before the dissemination of Mucoracease.
Contributing Institution:
National Institute of Animal Health, National Institute of Animal Health, National Agriculture and Food Research Organization (NARO)
3-1-5Kannondai, Tsukuba, Ibaraki 3050856, Japan
(WSC ID95)
http://www.naro.affrc.go.jp/english/niah/index.html
JPCDiagnosis:
- Abomasum: Abomasitis, necrotizing, multifocal to coalescing, marked, with vasculitis, thrombosis, and numerous mucosal and submucosal fungal pseudohyphae, hyphae, and yeasts.
- Abomasum, vessels: Vasculitis, necrotizing, multifocal, moderate with endothelial intranuclear viral inclusions.
JPC Comment:
The contributor has provided an excellent example of an endotheliotropic virus causing vascular damage, creating a permissive environment for the growth and invasion of multiple opportunistic fungal organisms, and setting the stage for systemic spread of Candida albicans. A remarkably versatile fungus, C. albicans has evolved numerous mechanisms of pathogenicity and adaptability that enable it to spread and cause disease in a wide range of host tissues.
C. albicans has three main morphologies: yeast, hypha, and pseudohypha. The yeast form exists at a lower pH and generally higher density than the hyphal form and is associated with fungal dissemination, whereas the hyphal form tends to invade tissue. A number of adhesins, including agglutinin-like sequence (Als) 3 and hyphal wall protein (Hwp) 1, facilitate adherence to tissue and formation of antibiotic and immunoresistant biofilms. C. albicans has two methods of invading cells. Surface integrins (including Als3) can interact with host ligands (such as E-cadherin) to stimulate endocytosis; or it can actively penetrate the cell through methods not fully elucidated.10
In order to survive in a variety of tissues with varied nutrient availability, C. albicans has developed metabolic adaptability. Glycolysis is its major source of energy in tissues rich in glucose (blood) or glycogen (liver), but in phagocytic cells such as macrophages, the fungus subsists on lipids and amino acids using gluconeogenesis and, later, the glyoxylate cycle. In other tissues, the fungus secretes proteases and subsists on liberated amino acids. 10
Interestingly, amino acid absorption indicates a less permissive environment, and, in response, the yeast will actively secreting ammonia to stimulate its own pH receptors and induce hyphal formation. The pH is detected using surface receptors Dfg16 and Rim21 which act through Rim101 transcription factor to mediate various cellular responses. In addition to switching morphologies, the fungus can respond to change in pH by altering expression of cell wall beta glycosidases Phr1 and Phr2 to maintain virulence in neutral-alkaline and acidic pHs, respectively.10
C. albicans has evolved a few mechanisms to obtain iron, another key nutrient for survival. While it does not produce its own siderophores, C. albicans absorbs iron from the siderophores of other microbes using the transporter Sit1. Alternatively, it can use Als3 to acquire iron from host ferritin and transferrin, or it may acquire iron from hemoglobin and heme using a variety of proteins.10
The moderator, Dr. Francisco (Paco) Uzal, discussed differentials for this case and highlighted the fact that bovine adenovirus 1 can cause identical clinical signs and intranuclear inclusion bodies as BAdV-4. In general, however, gastrointestinal disease caused by adenoviruses in cattle is rare.
References:
- Bianchi RM?Vielmo A?Schwertz CI et al. Fatal systemic Mortierella wolfii infection in a neonatal calf in southern Brazil. Cienc Rural. 2020;50(1):1-5.
- Chihaya Y?Furusawa Y?Okada H?Matukawa k?Matsui Y. Pathological Studies on Systemic mycoses in Calves. J Vet Med Sci. 1991;53?6?:1051-1058.
- Chihaya Y?Matsukawa K?Furusawa Y?Hatta Y?Okada H?Arisawa K. Disseminated aspergillosis with lesions in the central nervous system in a calf. Jpn J Vet Sci. 1988;50 (1):131-137.
- Chihaya Y?Okada H?Matsukawa K?Matsui Y. Disseminated mycoses in cattle. A study on nine autopsy cases. J Vet Med Sci. 1992;54?3?:485-491.
- Curtis B?Hollinger C?Lim A?Kiupel M. Embolic mycotic encephalitis in a cow following Mortierella wolfii infection of a surgery site. J Vet Diagn Invest. 2017;29(5):725-728.
- Davies JL?Ngeleka M?Wobeser GA. Systemic infection with Mortierella wolfii following abortion in a cow. Can Vet J. 2010;51:1391-1393.
- Matsui Y, Matsukawa K, Okada M, Chihaya Y, Kikuchi M. Bovine abortion by mycotic infection in Japan?particulaly isolation and identification of Aspergillus fumigatus and histopathological observations. Jap J Zootech Sci. 1977;48(9):481-488.
- Matumoto M, Inaba Y, Tanaka Y, Sato K, Ito H, Omori T. Serological typing of bovine adenovirus, Nagano and Fukuroi, as type 4 and new type 6. Jpn J Microbiol. 1969;13(1):131-132.
- Matumoto M, Inaba Y, Tanaka Y, Sato K, Ito H, Omori T. New Serological type 7 of bovine adenovirus. Jpn J Microbiol. 1970;14(5):430-431.
- Mayer FL, Wilson D, Hube N. Candida albicans pathogenicity mechanisms. 2013; 4(2): 119-128.
- Motes CM?Casares PC?Hundesa A?Martín M?Girones R. Detection of bovine and porcine adenoviruses for tracing the source of fecal contamination. Appl Environ Microbiol. 2004;70 (3):1448-1454.
- Munday JS, Laven RA, Orbell GMB, Pandey SK. Meningoencephalitis in an adult cow due to Mortierella wolfii. J Vet Diagn Invest. 2006;18:619-622.
- Nakagawa M, Hasegawa K, Suda H, Hiramune T. Bovine cerebellar zygomycosis? a case report. J Vet Med Sci. 1986;48?4?:809-812.
- Riet -Correa F, Krockenberger M, Dantas AFM, Oliveira DM. Bovine cryptococcal meningoencephalitis. J Vet Diagn Invest. 2011;23?5?:1056-60.
- Saini P, Singh M, Kumar P. Fungal endometritis in bovines. Open Vet J. 2019;9(1):94-98.
- Seyedmousavi S, Bosco SMG, Hoog SM, et al. Fungal infections in animals: a patchwork of different situations. Med Mycol. 2018;56:165-187.
- Thompson KG, Thomson GW, Henry JN. Alimentary tract manifestations of bovine adenovirus infections. Can Vet J. 1981;22:68-71.
- Uzai FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie MG ed. Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol.2 Elsevier; 2016:143-144.