Wednesday Slide Conference, Conference 7, Case 2
Signalment:
4-month-old, intact male mixed breed dog (Canis lupus familiaris).
History:
This animal had severe upper respiratory signs with green nasal discharge, severe conjunctivitis, lethargy, loss of appetite and dehydration.
Gross Pathology:
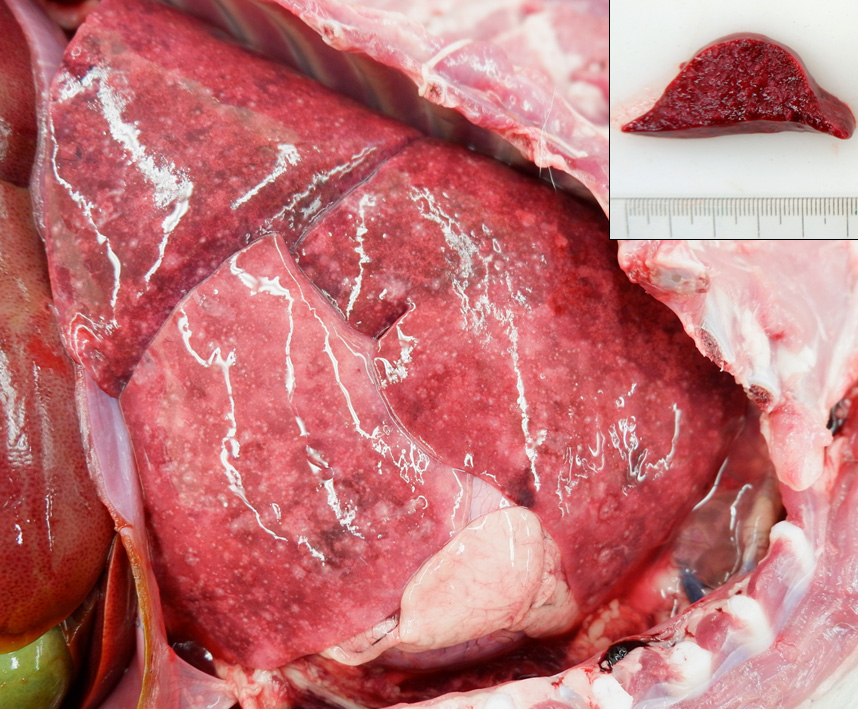
Examined is a 5.75 kg, juvenile (4-months-old) intact male mixed breed dog in good postmortem condition. A moderate amount of thin red-tinged transparent fluid exudes from the nasal and oral cavities. There is some mild frond-like proliferation of the skin on the dorsal nasal planum. The nasal turbinates are mostly reddened. There is negative pressure and approximately 125 mL of semitranslucent to slightly cloudy, thin, red-tinged fluid within the pleural cavity. The lungs fail to collapse, are diffusely dark red to purple, firm and contain innumerable pinpoint, firm, white nodules scattered throughout all lung lobes. A few sections float in formalin while a few sections sink. The tracheobronchial lymph nodes are subjectively enlarged and are diffusely dark red. The stomach contains a small amount of watery, tan fluid and the rugal folds are prominent. The intestinal tract contains a scant amount of tan watery ingesta. The colon is empty. The serosal surfaces of the intestinal tract have a subtle ground glass appearance.
Laboratory Results:
1. Aerobic culture (lungs): Escherichia coli (moderate growth), Staphylococcus pseudintermedius (moderate growth)
2. Canine Adenovirus (CAdV) 1 and 2 PCR (lung): Negative
3. Canine Distemper Virus (CDV) PCR (lung): Positive
4. Canine Herpesvirus 1 PCR (lung): Negative
5. Influenza A PCR (lung): Negative
Microscopic Description:
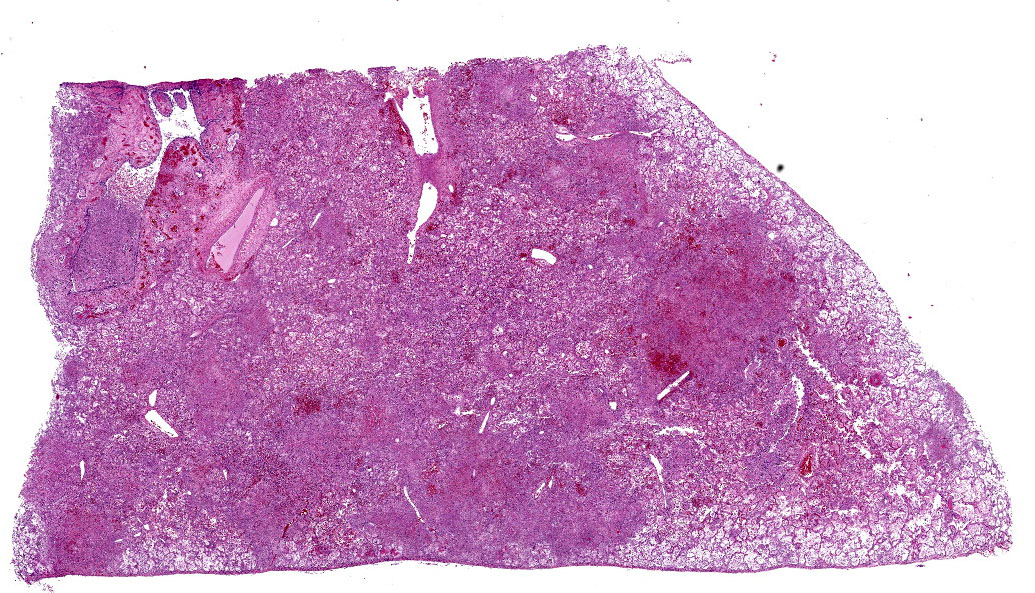
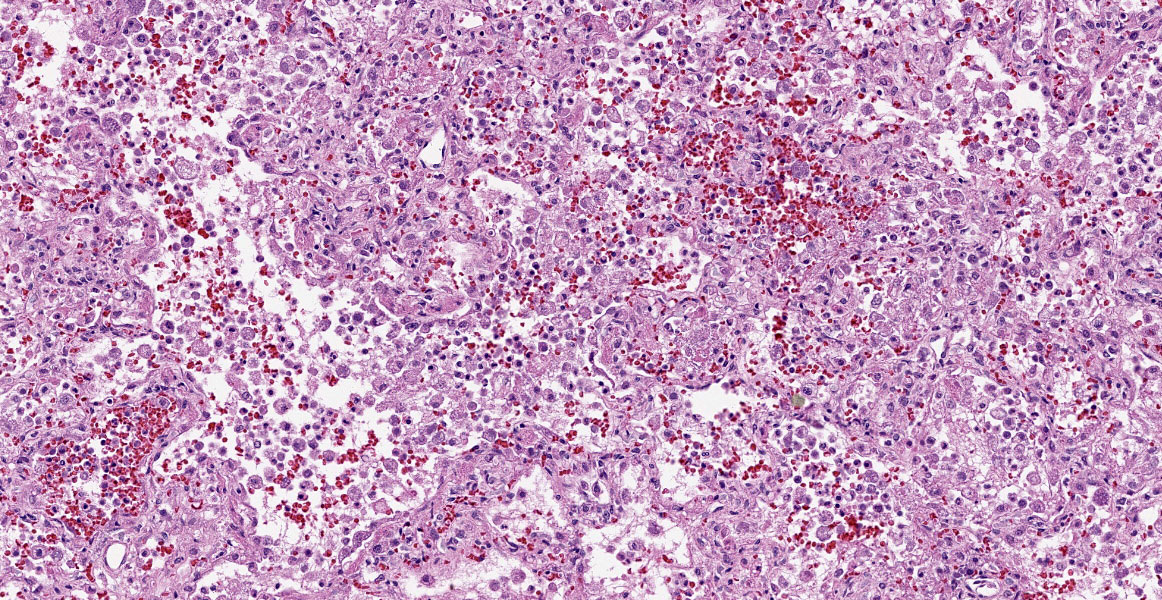
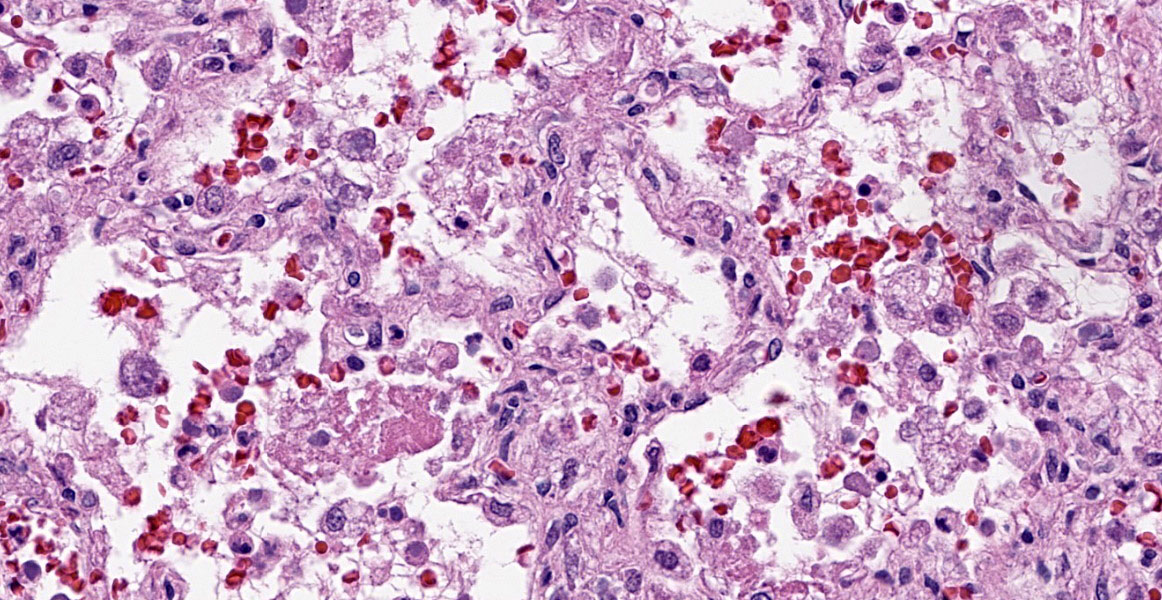
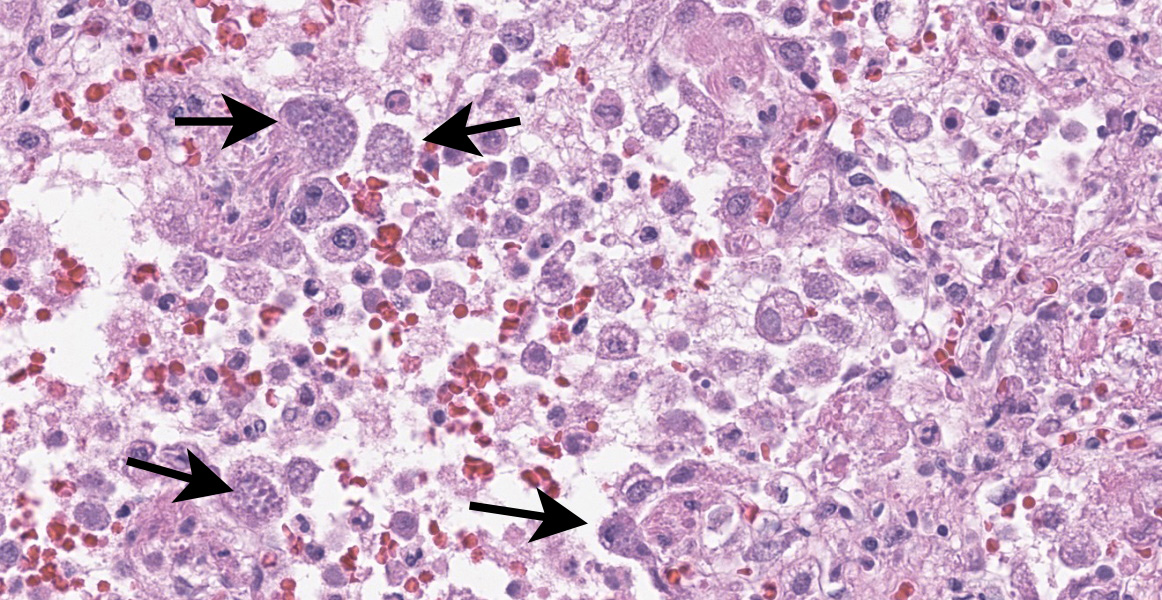
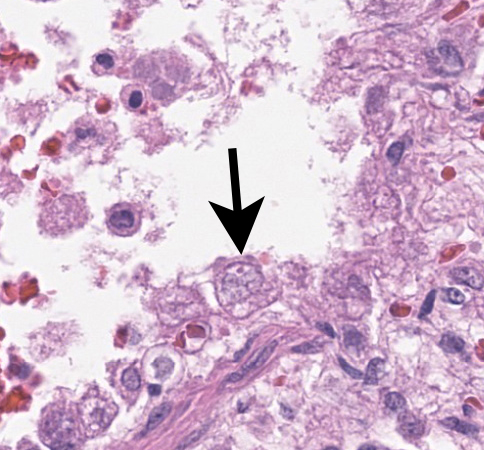
Lung: Multifocally and randomly scattered throughout the parenchyma are numerous loosely nodular aggregates of amorphous to granular eosinophilic debris (necrosis) that replaces the normal architecture. Areas of necrosis are characterized by accumulations of small basophilic granules (karyorrhectic debris), eosinophilic ghosts of necrotic round cells, extravasated erythrocytes (hemorrhage), abundant hypereosinophilic granular to fibrillar material (fibrin), and low numbers of degenerate macrophages. The adjacent alveolar spaces are often filled by plump and foamy macrophages, low numbers of neutrophils, and occasional sloughed degenerate epithelial cells mixed with hemorrhage, eosinophilic wispy material (edema) and fibrin. Scattered throughout examined sections and distending the cytoplasm of macrophages, alveolar epithelial cells or occasionally free within the tissues adjacent to disrupted macrophages are occasional to few, variably sized dense clusters of protozoal organisms. Organisms are round to fusiform, 2-4 µm in diameter and contain a small, central basophilic nuclear body. Rarely, viable and sloughed alveolar and bronchiolar epithelial cells contain small, eosinophilic intranuclear or intracytoplasmic viral inclusion bodies. Few bronchioles contain variable numbers of degenerate neutrophils and macrophages mixed with necrotic debris. The respiratory epithelium lining these bronchioles is often hypocellular and remaining epithelial cells are flattened (attenuated) or angular with moth-eaten cytoplasm and pyknotic nuclei (necrosis).
Immunohistochemical (IHC) staining of organisms in the lungs with an antibody targeting Toxoplasma spp. is performed. Numerous intracytoplasmic discrete clusters and scattered individual 2-4 µm diameter protozoal organisms exhibit positive immunostaining.
Other histopathology findings that are not included in the provided slide include:
- Hyperkeratosis of the nasal planum
- Lymphoplasmacytic conjunctivitis with rare viral inclusion bodies
- Toxoplasma organisms in the bone marrow, thyroid gland, and spleen
- Splenic lymphocytolysis
- Viral inclusions in mononuclear cells of mesenteric lymph node
Contributor’s Morphologic Diagnosis:
Lungs: Severe, multifocal to coalescing, necrotizing bronchointerstitial pneumonia with intranuclear and intracytoplasmic viral inclusion bodies and intracytoplasmic protozoal organisms consistent with Toxoplasma spp.
Contributor’s Comment:
Canine distemper virus (CDV) is caused by a Morbillivirus (family Paramyxoviridae)2 and has been shown to infect a wide variety of carnivores, including members of Canidae (domestic and wild dogs), Mustelidae (ferrets, mink), wild members of Felidae, raccoons6 and skunks.1 In addition to many terrestrial carnivores, some species of seals are also susceptible,2 including Caspian seals.8 In dogs, virus is transmitted through infected bodily tissues,12 particularly respiratory secretions.2 Within the upper respiratory tract, virus is phagocytosed by mucosal lymphocytes and macrophages, which travel to the tonsils and local lymph nodes. Here, the virus continues to replicate, infecting more lymphocytes and macrophages which then spread systemically.12 Within 2-5 days of exposure, the virus has spread throughout the body and can be found in various lymphoid tissues including bone marrow, thymus, and intestinal lymphoid tissues.2 Once within lymphoid tissues, the virus can then spread to and infect epithelial and mesenchymal (pantropic) cells throughout the body, particularly within the respiratory, nervous and alimentary systems.12
Within the respiratory tract, viral infection leads to death of pneumocytes, bronchiolar epithelium and macrophages, leading to impaired oxygen exchange, removal of debris and potential infectious organisms and decreased phagocytic and antigen-presenting ability by macrophages.12 Grossly, lesions of the respiratory tract include mucopurulent exudate within the nasopharynx as well as bronchointerstitial pneumonia characterized by patchy to diffuse red-tan, rubbery lesions,2 both of which are seen in this case. Histologically, mild suppurative inflammation within bronchioles as well as necrosis, attenuation of bronchiolar epithelium and intracytoplasmic (rarely, intranuclear outside of the nervous system) inclusion bodies may be observed.2
An important potential consequence of CDV infection is immune suppression and increased susceptibility to co-infections.2 Infection of and consequential cell death of lymphocytes and macrophages leads to lymphoid depletion of the thymus, lymph nodes, tonsils and various mucosal-associated lymphoid tissues. Various secondary infections have been documented in dogs including bacterial (Bordetella, Rhodococcus equi), viral (adenovirus), fungal (Pneumocystis, Cryptosporidium), and protozoal (Toxoplasma, Sarcocystis, Neospora).2,4,9,10 Immune suppression and secondary infections also play an important role in CDV infections in many other species with co-infections of Sarcocystis and Toxoplasma reported in raccoons and skunks,1,5,7 and Toxoplasma in mink, ferrets, and gray foxes.7
Toxoplasma gondii is an obligate intracellular coccidian protozoal organism9 that utilizes felid species as its definitive hosts but can likely cause disease in any mammal.11 Sexual reproduction only occurs within the feline gastrointestinal tract and infectious oocysts are released into the environment in feces. Transmission to other species occurs through ingestion of infected feces within the environment.11 In most immunocompetent hosts, Toxoplasma does not cause clinical disease, but with immune suppression particularly in young hosts, can lead to systemic toxoplasmosis. Dissemination of infectious tachyzoites occurs in various immune cells (lymphocytes, macrophages, granulocytes) or freely within the blood.11 Within the lungs, Toxoplasma infection is typically characterized grossly by small, white foci scattered throughout the parenchyma.2 Histologically, multifocal necrotizing interstitial pneumonia with histiocytic and neutrophilic infiltrates and Type II pneumocyte hyperplasia may be observed.6
Contributing Institution:
University of Illinois at Urbana-Champaign, Veterinary Diagnostic Laboratory
http://vetmed.illinois.edu/vet-resources/veterinary-diagnostic-laboratory/
JPC Diagnosis:
Lungs: Pneumonia, bronchointerstitial, fibrinonecrotizing and histiocytic, chronic, diffuse, severe with intranuclear and intracytoplasmic viral inclusions, and intracellular and extracellular protozoa.
JPC Comment:
This second case is a wonderful example of an interstitial pneumonia that is generous enough to have two separate entities present at the same time (figures 2-3 and 2-4)! We performed modified Gram stains (Brown-Brenn, Brown-Hopps) that confirmed the bacterial culture results for this case, particularly within the larger airways that contained necrotic debris and neutrophilic inflammation. Though this was a minor feature for this case, it fits with the overall immunosuppression related to canine morbillivirus that the contributor nicely outlines. Both Giemsa and PAS stains highlighted intrahistiocytic and intraalveolar cysts of Toxoplasma, though these were also readily apparent on H&E alone (figure 2-5). The presence of viral particles within select alveolar and bronchiolar epithelial cells was subtle (figure 2-6), though conference participants noted convincing intranuclear and intracytoplasmic inclusions. Conference participants also discussed whether there were viral syncytial cells present but were not confident that these could be distinguished from multinucleated giant cell macrophages. One takeaway from this case is that necrotizing pneumonias where Toxoplasma is suspected should prompt a careful search for an inciting cause for immunosuppression with canine morbillivirus having recognizable features in section.
There are several major risk factors for canine toxoplasmosis. As the contributor notes, primary disease is associated with immunosuppression.3 Seroprevalence estimates from around the world vary, though national estimates vary from 7.9% to 42.8% of dogs.3 Cohabitation of dogs with cats (the definitive host) is associated with greater seroprevalence as is outdoor housing of dogs and coprophagy habits. With these data in mind, exposure of dogs to Toxoplasma is a likely event in many environments though immunocompetent animals are typically subclinically infected and do not exhibit signs. The role of vaccination and the possibility of diminished maternal immunoglobulins from poor transfer or lack thereof is worth noting for this case, though the history for this animal is sparse.
References:
1. Burcham GN, Ramos-Vara JA, Vemulapalli R. Systemic Sarcocystosis in a Striped Skunk (Mephitis mephitis). Veterinary Pathology. 2010;47(3):560-564.
2. Caswell JL, Williams KJ. Respiratory System. ln: Maxie MG ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol. 2. 6th ed. Philadelphia, PA: Elsevier Saunders. 2016: 574-576, 590.
3. Dini FM, Stancampiano L, Poglayen G. et al. Risk factors for Toxoplasma gondii infection in dogs: a serological survey. Acta Vet Scand. 2024;66(14).
4. Headley, SA, Oliveira TES, Pereira AHT, Moreira JR, et al. Canine morbillivirus (canine distemper virus) with concomitant canine adenovirus, canine parvovirus-2, and Neospora caninum in puppies: a retrospective immunohistochemical study. Nature Scientific Reports.
5. Kubiski SV, Sisó S, Church ME, Cartoceti AN, Barr B, Pesavento PA. Unusual Necrotizing Encephalitis in Raccoons and Skunks Concurrently Infected with Canine Distemper Virus and Sarcocystis sp. Veterinary Pathology. 2016;53(3):674-676.
6. Lopez A. Respiratory System, Mediastinum and Pleurae. Ln: McGavin MD, Zachary JF eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby Elsevier; 2012: 524-527.
7. Moller T, Nielsen SW. Toxoplasmosis in Distemper-Susceptible Carnivora. Pathologia veterinaria. 1964; 1(3): 189-203.
8. Namroodi S, Shirazi AS, Khaleghi SR, N. Mills J, Kheirabady V. Frequency of exposure of endangered Caspian seals to Canine distemper virus, Leptospira interrogans, and Toxoplasma gondii. PLoSONE. 2018;13(4).
9. Portilho FVR, Paes AC, Megid J, Hataka A, et al. Rhodococcus equip VAPN type causing pneumonia in a dog coinfected with canine morbillivirus (distemper virus) and Toxoplasma gondii. Microbial Pathogenesis. 2019; 129: 112-117.
10. Postma GC, Dellarupe A, Streitenberger N, Bratanich A, et al. Canine distemper virus, atypical Toxoplasma gondii, and Neospora caninum co-infection, in a dog with neurological signs from Argentina. Brazilian Journal of Veterinary Pathology. 2019; 12(3): 101-105.
11. Uzal FA, Plattner BL, Hostetter JM. Alimentary System. ln: Maxie MG ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol. 2. 6th ed. Philadelphia, PA: Elsevier Saunders. 2016: 236-237.
12. Zachary JF. Mechanisms of Microbial Infections. Ln: McGavin MD, Zachary JF eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby Elsevier; 2012: 226-227.