Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 4
Sept 19, 2018
CASE II: NA (JPC 4066460-00).
Signalment: Adult (>2.5 years) male cynomolgus monkey, Macaca fascicularis
History: This study was conducted in accordance with the current guidelines for animal welfare. All procedures performed on these animals were in accordance with regulations and established guidelines and were reviewed and approved by the Institutional Animal Care and Use Committee. For further information, please see Contributor’s Comments.
Gross Pathology: No macroscopic findings
Laboratory results:
Clinical pathology findings:
Increased from pre-study baseline: AST, Urine volume, Urine protein and protein ratio, Urine blood, Urine creatinine, Urine NAG, and Urine microalbumin
Decreased from pre-study baseline: GGT, Total protein, Albumin, Ca
Light microscopic findings:
Glomerulus: Moderate mesangial and podocyte hyperplasia, mild eosinophilic inclusions in podocytes, mild granulocytes in capillary lumens, marked increase in mesangial matrix, moderate synechia, moderate parietal epithelial cell hyperplasia, marked C5b-9 granular staining of capillary walls and podocytes (IHC)
Tubules: Mild distal tubular dilation, minimal distal tubular luminal hyaline casts, moderate erythrocyte casts in proximal and distal tubular lumens
Ureter: Minimal lumenal erythrocytes
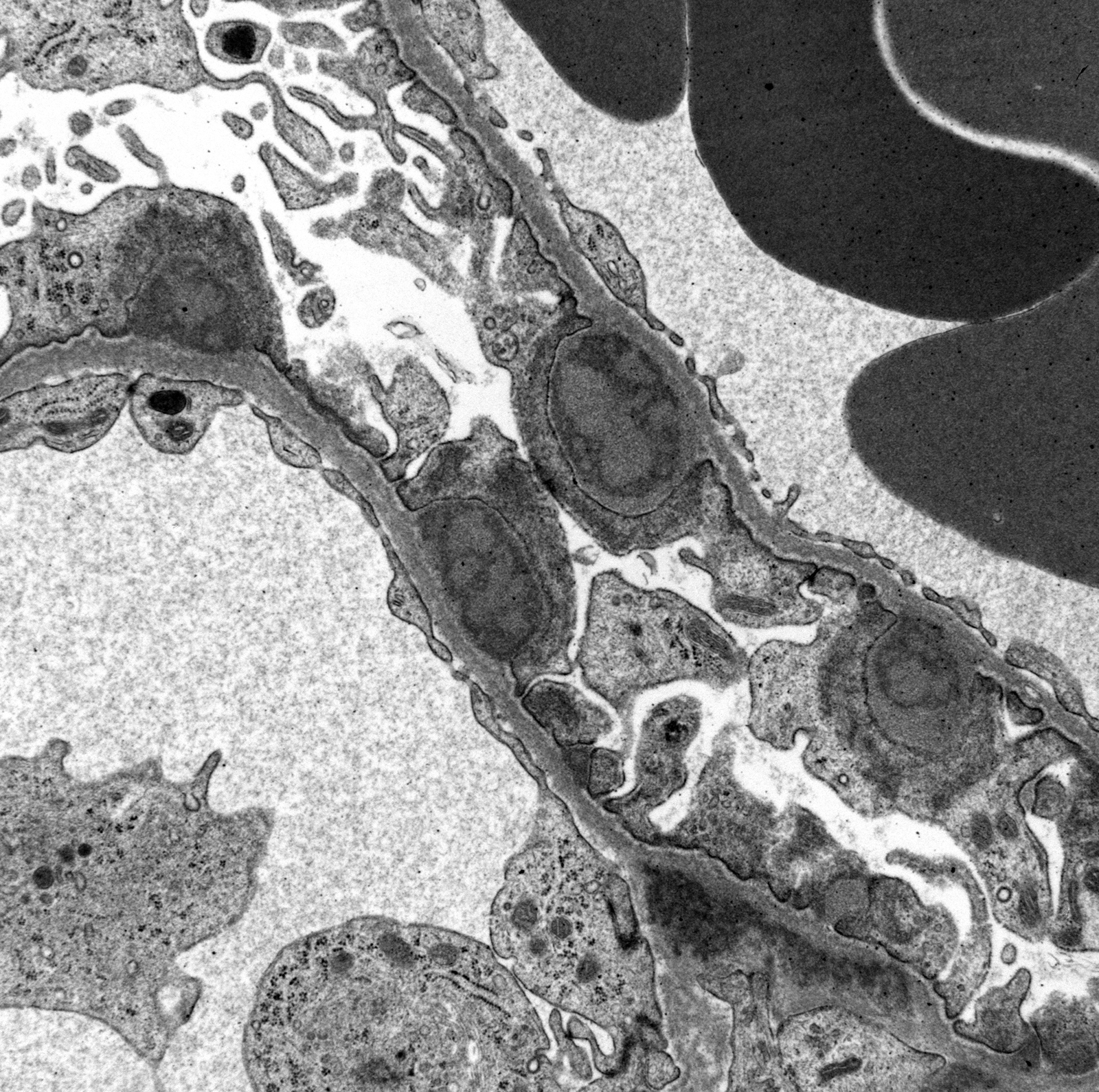
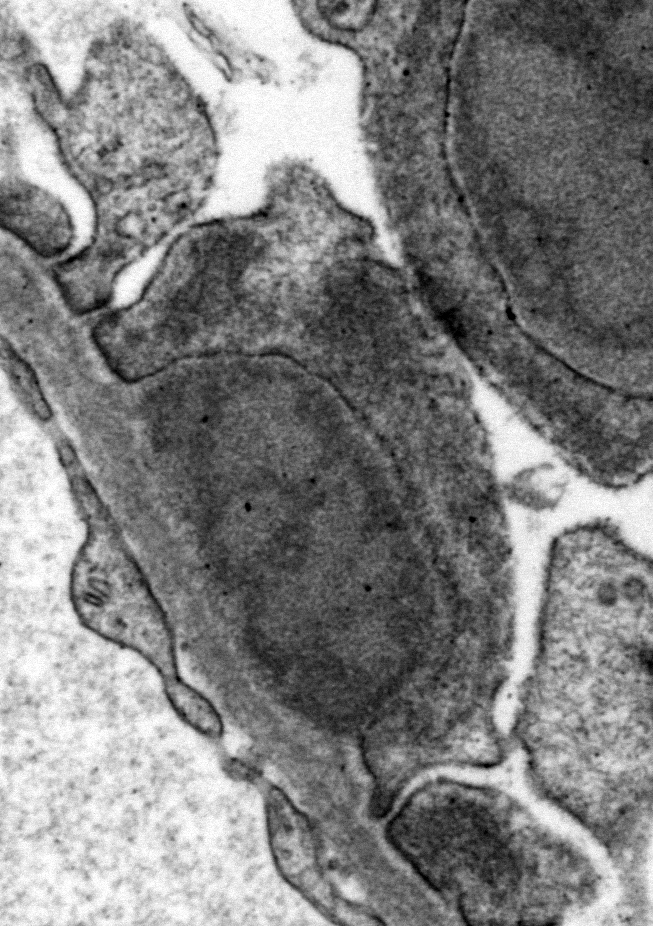
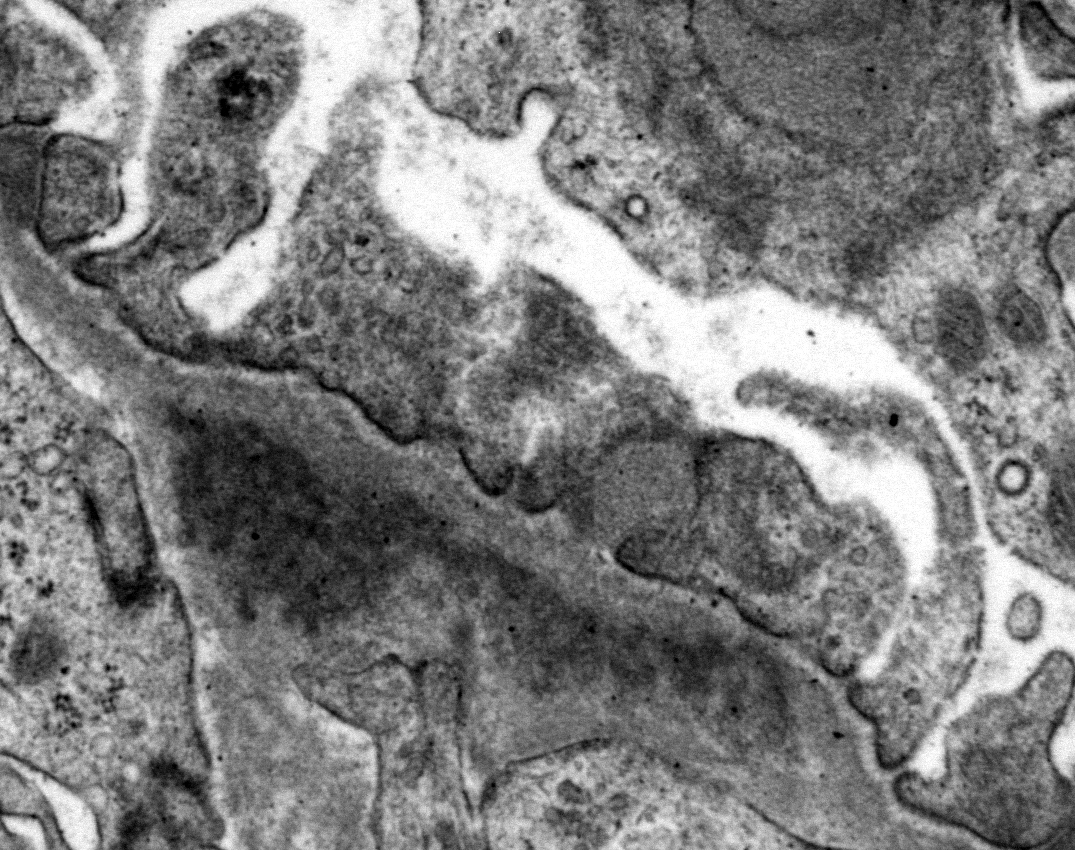
Ultrastructural Description: Kidney, portions of two glomerular capillary loops (5,000x original negative magnification) -There are four large generally similar, approximately 500-700 nm diameter, oval-to-spherical, subepithelial, electron dense deposits morphologically consistent with immune complexes. Each consists of an irregular mixture of darker and less dark areas. Facing the capillary lumen, these deposits contact the lamina rara externa of the glomerular basement membranes, and facing the urinary space, deposits contact and are encircled by fused podocyte foot processes (foot process effacement). Podocyte foot process cytoplasm adjacent to these deposits contains flocculent electron dense material. One smaller subepithelial roughly-triangular dense deposit is also present, and there is also an irregular intramembranous electron dense deposit on one border of the image. Podocyte foot processes adjacent to this intramembranous deposit and in capillary loop sites not adjacent to deposits are also effaced, but less severely so.
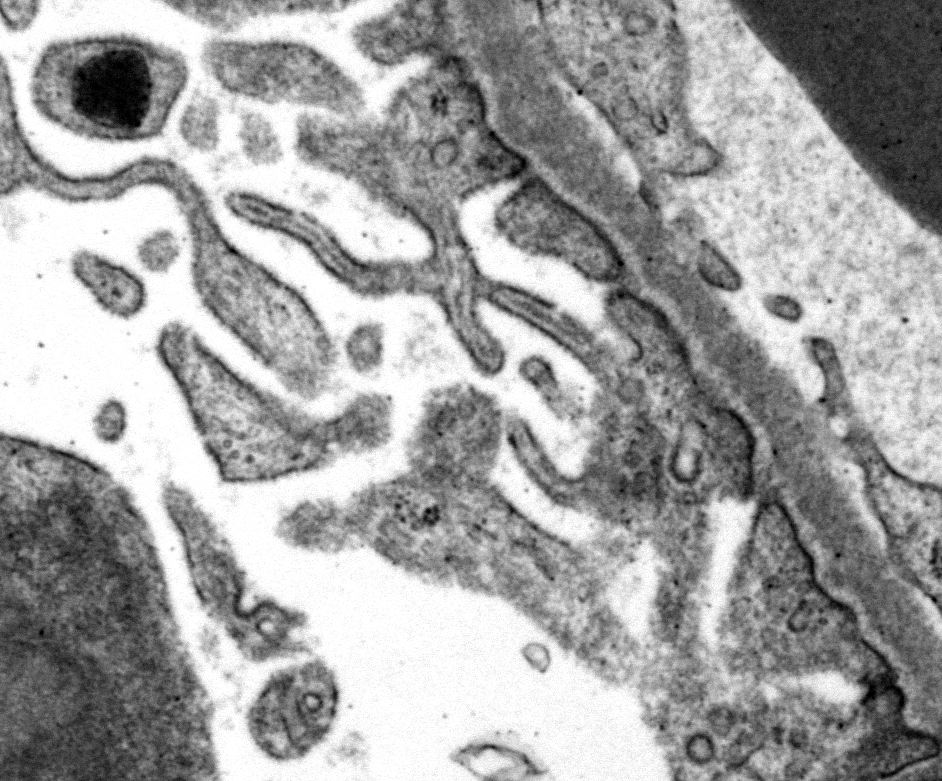
There are narrow branching cytoplasmic projections extending from podocytes into the urinary space (podocyte hypertrophy), light accumulations of flocculent material in the urinary space (interpreted as protein leakage), and a circular intensely electron dense spherical inclusion in one podocyte pedicle (interpreted as resorbed protein). Glomerular capillary endothelium of one capillary lacks most of its fenestrations, and endothelial cells lining this capillary are enlarged and protruding into the capillary lumen.
Contributor’s Morphologic Diagnoses: Kidney: Glomerulopathy, with subepithelial and intramembranous electron dense deposits, podocyte foot process effacement, and podocyte hypertrophy
Contributor’s Comment: The overall experimental design employed in this study was adapted from that described by Hebert,4 In that study, animals administered bovine gamma globulin (BGG) intravenously at 5.5 mg/kg/day (dosed five days per week) developed glomerulopathy beginning as early as after six weeks of dosing. In a previous pilot study conducted by the comtributor, the methods and dose levels outlined below successfully induced immune complex-mediated glomerulopathy. This animal was immunized with, and then dosed with, BGG according to the schedule outlined in the table below.
Immunization: On pre-study Day -48, 1.3 mL of Complete Freunds Adjuvant (CFA) at 0.5 mg/mL was combined with 1.3 mL of BGG, reconstituted in PBS, formulated to deliver 1 mg/kg, and administered subcutaneously at 10 different sites along the upper portion of the dorsal thorax. Total volume administered was 2 mL. On pre-study Day -22, 1.3 mL of Incomplete Freunds Adjuvant (IFA) was combined with 1.3 mL of BGG reconstituted in PBS, formulated to deliver 1 mg/kg, and administered subcutaneously at 10 different sites along the upper portion of the dorsal thorax. Total volume administered was 2 mL. All animals were anesthetized with ketamine for CFA and IFA administration and given buprenorphine.
Antigen dosing: BGG was administered once daily over 30 minutes through a vascular access port. On each BGG dosing day, 5 mg/kg of diphenhydramine (DPH) was administered intramuscularly approximately 15-30 minutes prior to BGG administration.
Day 1 |
Day 2 |
Day 3 |
Day 4 |
|
5 mg/kg DPH 0.5 mg/kg (0.1 mg/mL) BGG |
5 mg/kg DPH 0.5 mg/kg (0.1 mg/mL) BGG |
5 mg/kg DPH 1.0 mg/kg (0.2 mg/mL) BGG |
5 mg/kg DPH 1.0 mg/kg (0.2 mg/mL) BGG |
|
Day 5 |
Day 6 |
Day 7 |
Day 8 to Study End |
|
5 mg/kg DPH 2.0 mg/kg (0.4 mg/mL) BGG |
5 mg/kg DPH 3.0 mg/kg (0.6 mg/mL) BGG |
5 mg/kg DPH 4.0 mg/kg (0.8 mg/mL) BGG |
5 mg/kg DPH 5.5 mg/kg (1.1 mg/mL) BGG |
BGG immunization followed by daily BGG dosing was tolerated with antihistamine pre-treatment and induced proteinuria and immune complex glomerulopathy in this animal after 8 weeks.
JPC Diagnosis: Kidney: Glomerulonephritis, membranous, diffuse, marked, with subepithelial dense deposits, effacement of podocyte foot processes, and podocyte villar hypertrophy.
Conference Comment: This outstanding electron micrograph excellently demonstrates immune complex deposition in subepithelial areas and effacement of the overlying podocytes. At the bottom corner of the image, a more traditional appearance of dense deposits is seen, which do not result in the bulging of the basement membrane; however podocytes overlying these deposits are similarly effaced. It also demonstrates the uncommon lesion of villous transformation of podocytes, which is usually seen in advanced stages of podocyte effacement.
Immune complex glomerulonephritis (ICGN) is the classic type III hypersensitivity reaction resulting from long-standing inflammation, formation of large amounts of antigen-antibody complexes in circulation, and their deposition within the glomerular basement membrane (GBM). The offending antigen may be that of an infectious organism causing a chronic infection (i.e. D. immitis) or may be endogenous in nature (as is seen in systemic lupus erythematosus in humans). 1 Other non–ICGN types of glomerulonephritis include anti-GBM disease as well as those forms which fix complement.1 This particular model, of periodic injections of bovine serum albumin, mimics the continued antigenemia of infectious disease.
The basic theory of ICGN is somewhat controversial, as many animals with circulating soluble immune complexes may present without glomerulonephritis; others believe that the deposition of immune complexes may be secondary to previous glomerular injury.1 Additionally other molecules such as C3, capital C1q, and IgM may also adherent to previously injured tissue.1
Localization of immune complexes are generally described as subendothelial, subepithelial, intramembranous, and mesangial. Their localization within the GBM is predicated on a number of factors including their size, shape, charge, and chemical composition as well as the presence of local mediators of inflammation which may affect transport across endothelium or within the basement membrane.1 A number of features may also impact the persistence of immune complexes within the basement membrane. Phagocytosis by neutrophils or macrophages, removal of the source of the persistent antigenemia, extracellular degradation by proteases, and even egress from the basement membrane may result in elimination.1 At the other end of the spectrum, immune complexes may enlarge within the basement membrane through addition of small amounts of antigen or antibody, complement, or by addition of similar immune complexes.1
A recently published classification of glomerular disease in the dog2 identified immune complex-mediated glomerulonephritis as one of the two large categories of glomerular disease, with the other major category characterized by the absence of immune complexes and largely composed of cases of glomerular amyloidosis or focal segmental glomerulosclerosis. When examined only by light microscopy, 22/89 cases were misdiagnosed, emphasizing the importance of additional diagnostic modalities (especially electron microscopy) beyond light microscopy alone.2
The WSAVA Classification denotes the characteristic subepithelial location of ICs in cases of membranous glomerulonephritis (as seen in this case), and subendothelial or mesangial locations in cases of membranoproliferative glomerulonephritis. The subepithelial location of the immune complexes results in characteristic glomerular changes. The interposition of basement membrane between the ICs and the capillary lumen does not engender endocapillary hypercellularity (an increase in the number of leukocytes, endothelial cells and interposed mesangial cells internal to the GBM) which ultimately encroaches or obliterates the lumina of glomerular capillaries.2 The ability of these subepithelial complexes to fix complement, however, does result in effacement of podocyte foot processes.2 Conversely, subendothelial locations of ICs results in activation of endothelium and transcapillary recruitment of inflammatory cells and peripheral capillary hypercellularity as previously described.2
Interestingly, reports of ICGN in nonhuman primates are relatively scarce within the last decade. Membranoproliferative glomerulonephritis with crescent formation was reported in cynomolgus macaques injected with obinutuzumab, a monoclonal antibody targeted against CD20,5 exemplary of the immunogenicity of foreign antibodies in primate models. Membranoproliferative glomerulonephritis was also attributed to chronic SHIV infection in a rhesus macaque.3
A potential differential on ultrastructure for the immune complexes would in this case would be “hump-like” deposits of complement, as seen in post-infectious glomerulonephritis in humans with a history of recent infectious disease. These “humps’ are primarily composed of the third component of complement with little immunoglobulin present.
Contributing Institution:
Pfizer
588 Eastern Point Rd
Groton, CT 06340
References:
- Cianciolo RE and Mohr FC. Urinary System. In: Maxie MG, ed. Jubb Kennedy and Palmer’s Pathology of Domestic Animals, 6th 2016; St. Louis MO, Elsevier Press, vol 2, p. 411-412.
- Cianciolo RE, Mohr FC, Aresu L, Brown CA, James C, Jansen JH, Spangler WL, van der Lugt JJ, Kass PH, Brovida C, Cowgill LD, Heiene R, Polzin DJ, Syme H, Vaden SL, van Dongen AM, Lees GE. World Small Animal Veterinary Association Renal Patholgy Initiative: Classification of Glomerular Diseases in Dogs. Vet Pathol 2016; 53(1): 113-135.
- Clarke CL, Eckhaus MA, Zerfas PM, Elkins WR. Peripheral edema with hypoalbuminemia in a nonhuman primate infected with simian-human immunodeficiency virus: a case report. J Am Assoc for Lab Anim Sci 2008; 47(1)42-48.
- Hebert L, Cosio F, Birmingham D. Experimental immune complex-mediated glomerulonephritis in the nonhuman primate. Kid Intern. 1991; 39:44-56.
- Husar E, Solonets M, Kuhlmann, O, Schick E, Piper-Leputre H, Singer T, Tyagi G. Hypersensitivity reactions to obinutuzumaba in cynomolgus monkeys and relevance to humans. Toxicol Pathol 2017; 45(5)676-686.