Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 3
Sept 12, 2018
CASE II: AFIP 15-13332 (JPC 4067411-00).
Signalment: 8-month-old Romney ewe lamb (Ovis aries)
History: The ewe lamb was found recumbent and unable to stand. Paddling movements and spasms were observed prior to euthanasia.
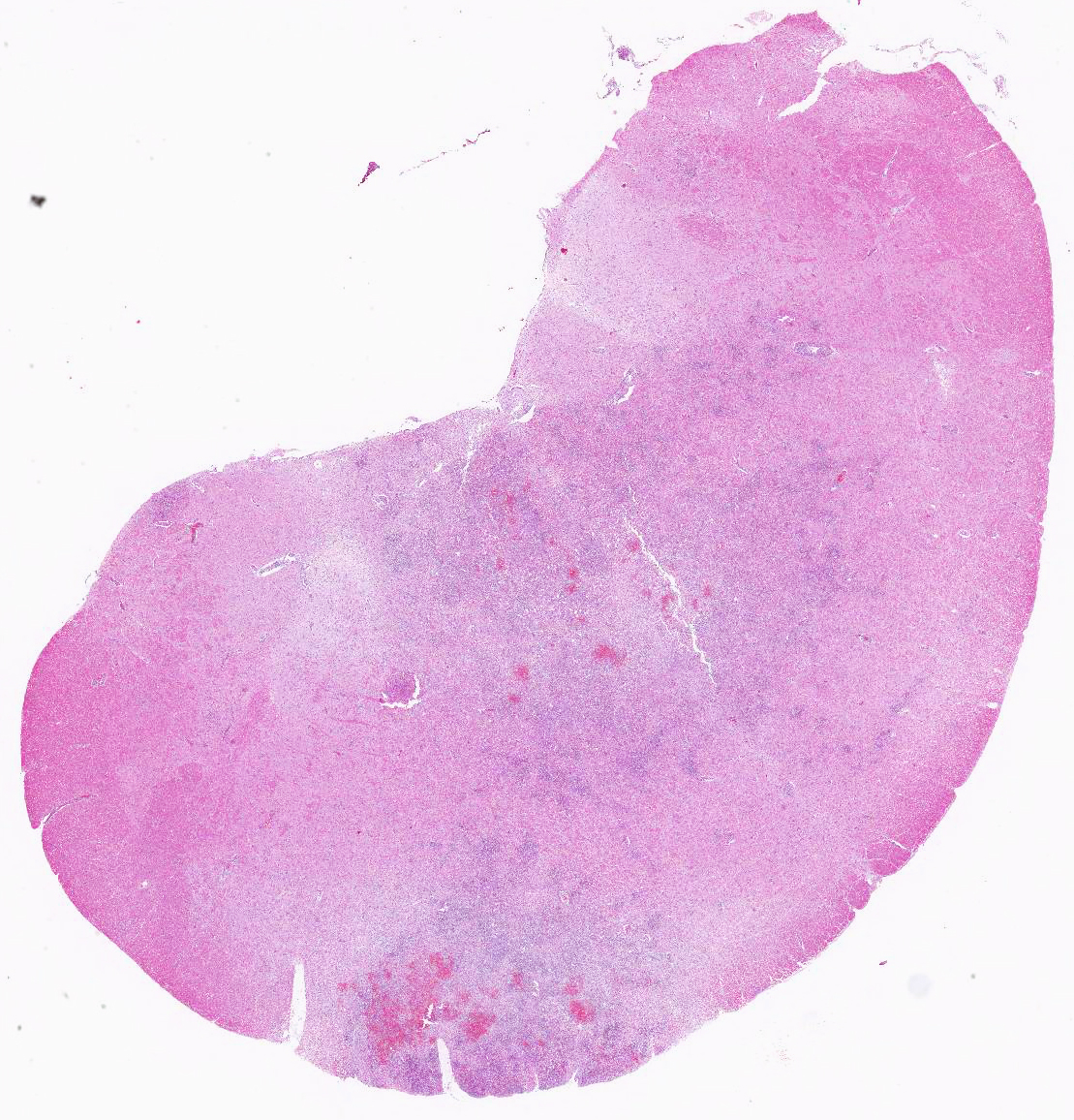
Gross Pathology: The 8-month-old Romney ewe lamb was in good body condition (BCS 3/5), with good muscle mass, fat reserves, and adequately hydrated. Numerous variably-sized (2 mm-5mm) areas of hemorrhage were present in the medulla oblongata and extended caudally to the level of the obex and cranial cervical spinal cord. No other gross lesions were present elsewhere.
Laboratory results: None.
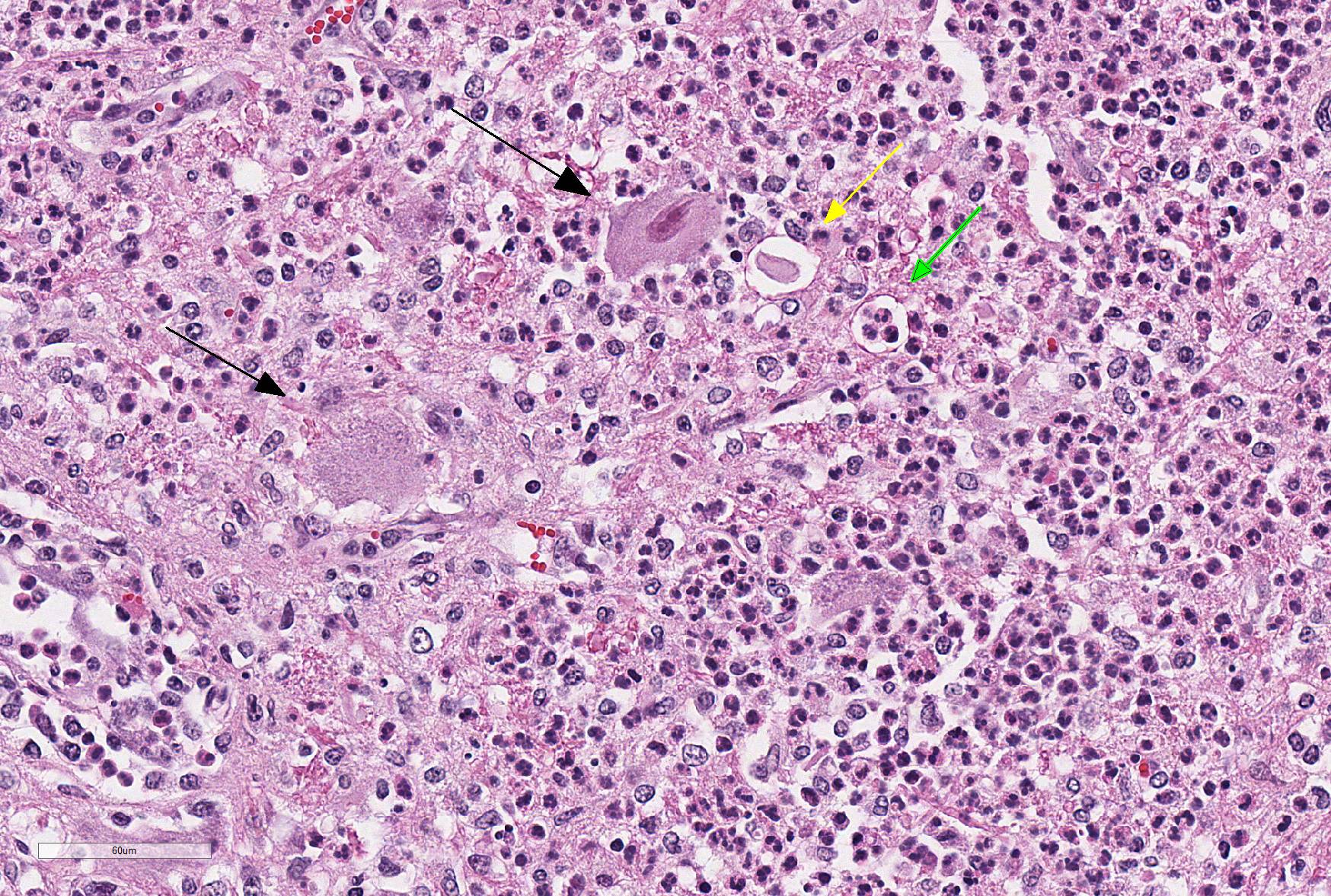
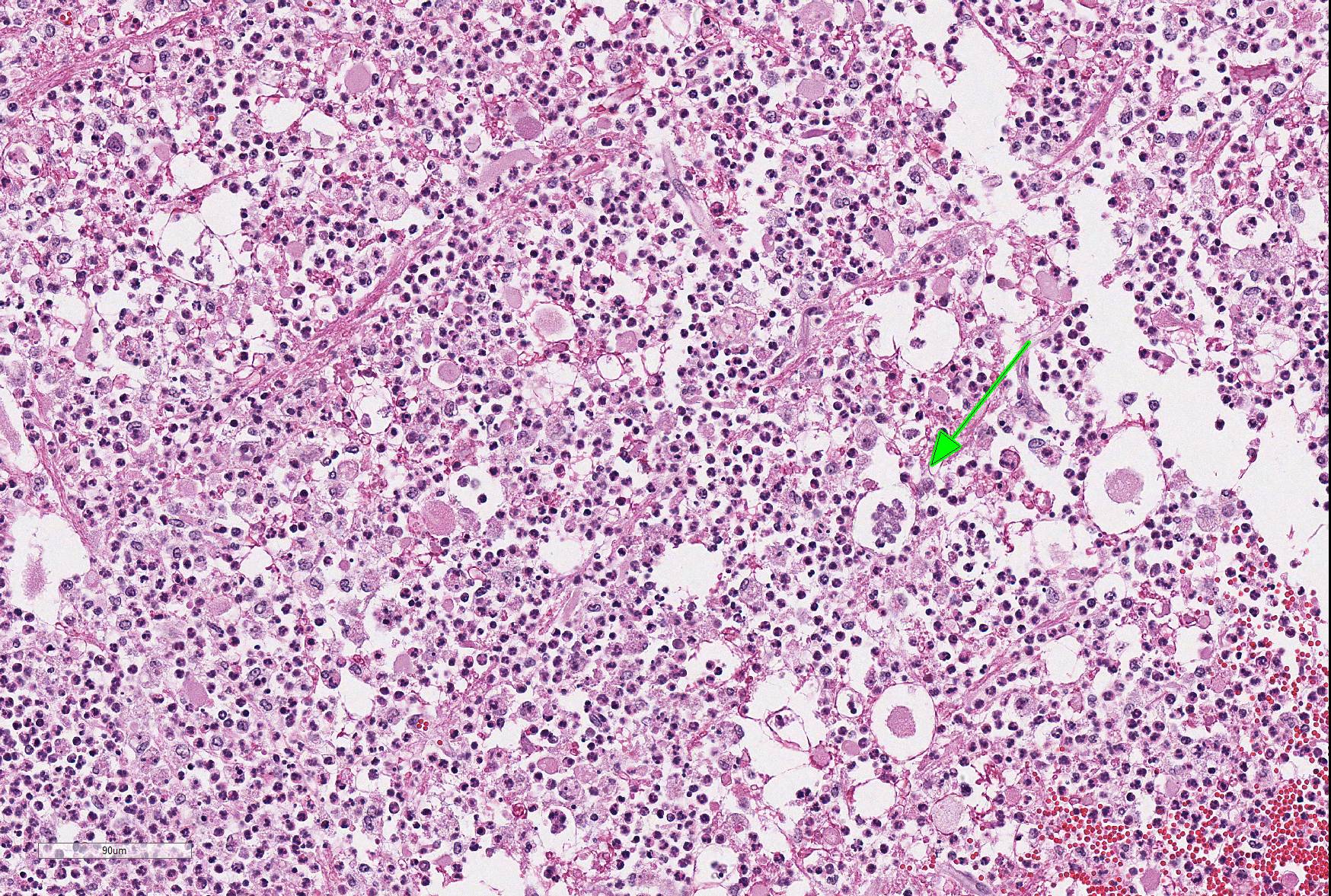
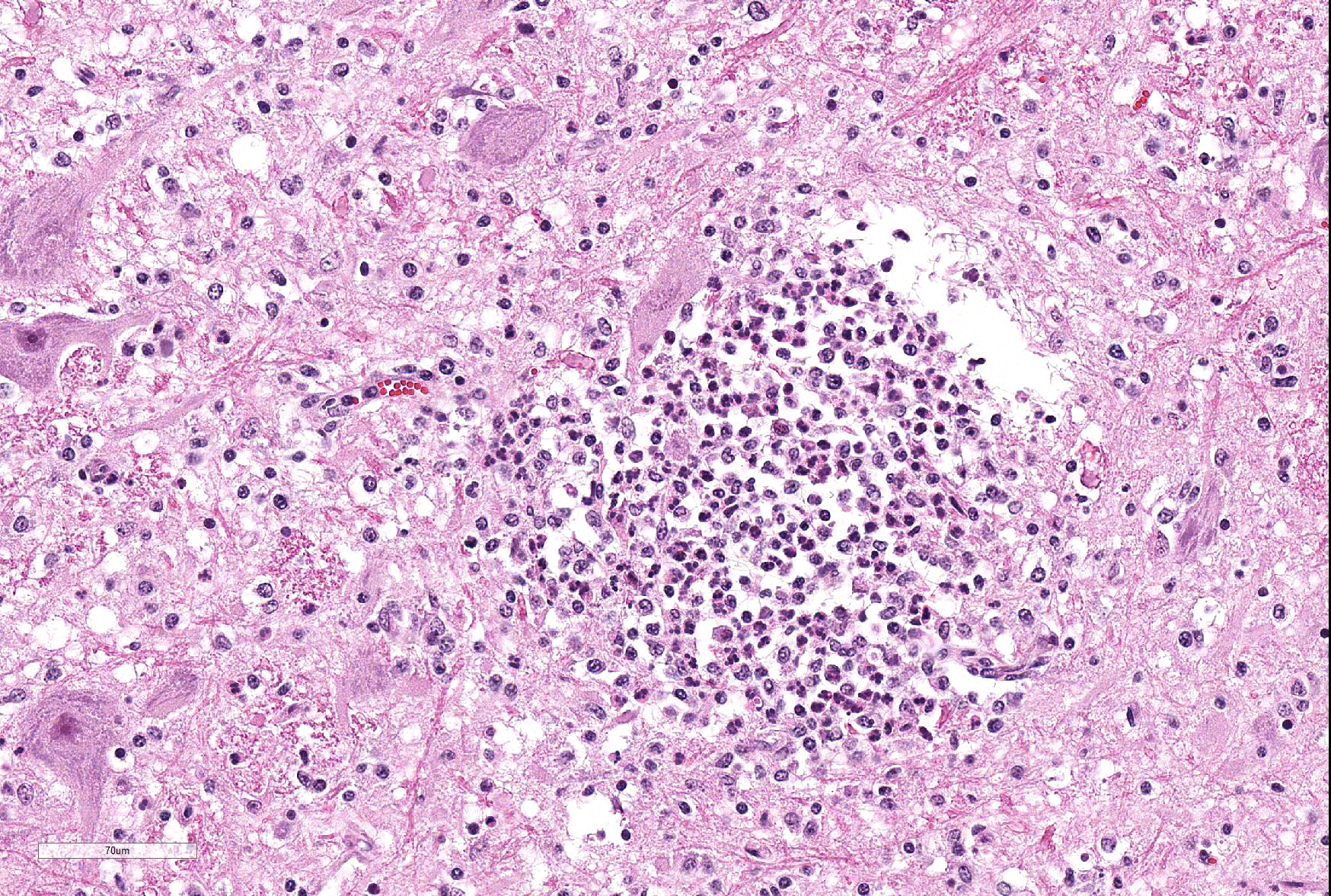
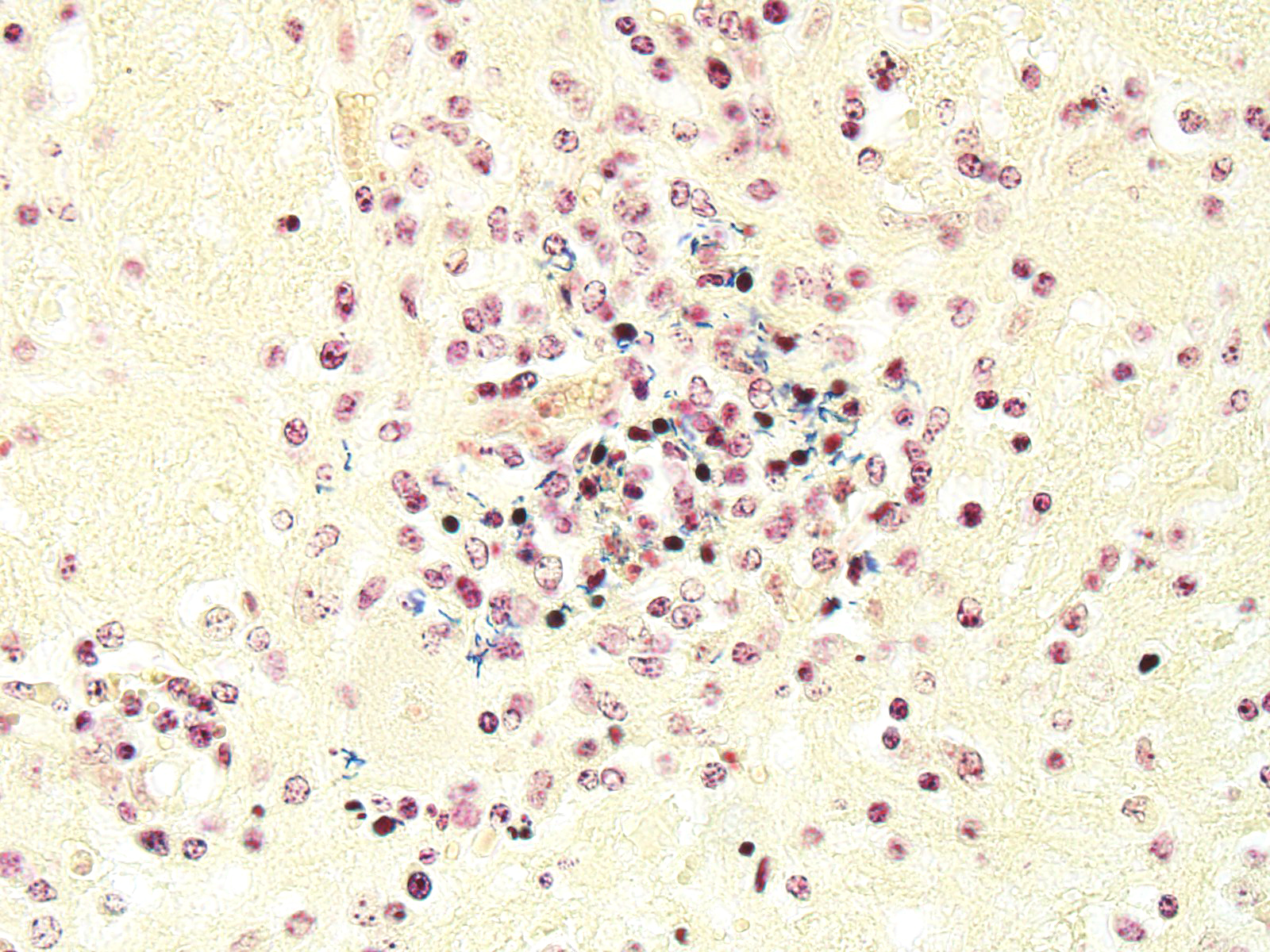
Microscopic Description: Within the brainstem and affecting both gray and white matter, are multifocal to coalescing, variably-sized aggregates of numerous intact and degenerate neutrophils and lesser numbers of macrophages and gitter cells (microabscesses) surrounded by small areas of gliosis. Within and adjacent to the areas of inflammation, numerous axons are swollen and eosinophilic (spheroids) and rare hypereosinophilic, shrunken neurons with pyknotic nuclei surrounded by neutrophils are present (neuronal necrosis and neuronophagia). Multifocally, the white matter is edematous and rarefied. Within the parenchyma, there are numerous densely cellular perivascular cuffs mainly composed of lymphocytes and plasma cells, with lesser number of macrophages, neutrophils and rare eosinophils. A Gram stain reveals moderate numbers of predominantly intracellular, short, plump gram-positive coccobacilli. Additionally, there are multifocal areas of hemorrhage in the parenchyma and locally extensive regions of leptomeningeal hemorrhage. Multifocally, lymphocytes and plasma cells, and lesser numbers of neutrophils and macrophages, surround and disrupt meningeal blood vessels.
Contributor’s Morphologic Diagnoses: Brainstem. Meningoencephalitis, necrosuppurative, subacute, multifocal to coalescing with gram-positive coccobacilli (Listeric encephalitis)
Contributor’s Comment: The clinical signs and the microscopic findings in the brain of this ewe lamb are consistent with listeric encephalitis.1-6,9 Listeria monocytogenes belongs to the bacterial genus Listeria, which are gram-positive, non-spore-forming, facultative anaerobic, intracellular coccobacilli ubiquitously found in the environment and commonly present in the faeces of healthy animals.1,3,5,6 It has been suggested that domesticated ruminants play a key role in the maintenance of Listeria spp. in the environment through a continuous faecal–oral enrichment cycle.1 There are currently six species in the genus Listeria, but only L. monocytogenes and L. ivanovii are considered pathogenic for domestic animals.5-7
Virulent strains possess a group of virulence genes such as the positive regulatory factor A, internalins, hemolysins (such as listeriolysin O), phospholipases, a hexose phosphate transporter and others, which allow them to replicate within and spread between eukaryotic cells.1,4-8 Critical to the virulence of Listeria, is the ability to escape intracellular killing within macrophages by lysis of the phagosomal membrane and escape into the cytoplasm, actions mediated by the secretion of listeriolysin O.5-7 The importance and role of these genes in neurovirulence and neuroinvasion in natural infections, whether by hematogenous infection or axonal migration, is yet to be determined.6-7
In ruminants, the main clinical features of Listeria infection are encephalitis, septicaemia, abortion, mastitis and gastroenteritis.1-6,9 Listeriosis in ruminants occurs primarily in sporadic cases and is considered to be non-contagious.1,5 In general, the disease is more frequent in winter and early spring and outbreaks of disease are known to occur when animals are exposed to a single contaminated source such as silage.1,3-6 However, outbreaks of listeric encephalitis have been described unrelated to silage feeding.8-9 Pathogenesis of listeric encephalitis in sheep, goats and cattle suggests that L. monocytogenes gains access to the brainstem via migration through axons of various cranial nerves (most commonly the trigeminal nerve), using the oropharyngeal mucosae as the port of entry.6-8 Once the bacteria has accessed the CNS it spreads further from the brainstem into other brain regions probably by intracerebral axonal migration.7-8 Clinical signs of listeric encephalitis are similar in ruminants and vary depending on the topography of the CNS lesions.1-3 The neurologic signs of listeriosis in sheep reflect dysfunction of caudal brainstem, cerebellar peduncles and/or cranial nerves III through XII.1-3 Gross lesions of the brain are generally absent, or limited to mild meningeal congestion, unilateral or bilateral areas of malacia in the brainstem or spinal cord, and mild clouding of the cerebrospinal fluid.1,3,5-8 In this case the gross lesions consisted of variably-sized areas of hemorrhage in the medulla oblongata that extended caudally to the level of the obex and cranial cervical spinal cord. Histologically, microabscesses, focal gliosis with adjacent perivascular cuffs consisting predominantly of lymphocytes with histiocytes, plasma cells and occasional neutrophils characterize listerial encephalitis.1,6-8 In severe cases, such as the present case, lesions may coalesce to affect large areas of brain tissue.7 Meningitis is often present, and thought to develop secondary to parenchymal lesions.5-7 Species differences can be observed in lesion distribution throughout the CNS, and in the extent and cellular composition of the inflammatory reactions.7 Although the characteristic lesion is the presence of microabscesss both in cattle and small ruminants, cattle tend to have relatively more macrophages and more prominent mononuclear perivascular cuffs than small ruminants. Neuronal necrosis appears to be more frequent in small ruminants than in cattle.7
In this case, bacterial culture was not attempted since the clinical presentation, together with the gross and histological lesions, and the demonstration of gram-positive, intracellular coccobacilli in histological sections, were consistent with Listeria infection.
JPC Diagnosis: Brainstem: Rhombencephalitis, necrotizing and suppurative, multifocal to coalescing, severe, with microabscesses and mild lymphohistiocytic meningitis.
Conference Comment: The contributor has none an excellent job in describing the general aspects, virulence factors, and pathogenesis of the encephalitic form of listeriosis in small ruminants. A number of other syndromes have been identified in small ruminants infected with Listeria monocytogenes, to include enteritis, septicemia, abortion, mastitis, and ophthalmitis. In addition, the contributor has correctly utilized the various, often non-intuitive spellings of the tissue Gram stain, named after Dr. Hans Christian Gram, who developed the method in 1884. When describing the particular stain, the upper case “Gram” is used – “She performed a Gram stain on each section”. When describing the stain’s positivity or negativity, the lowercase version is used – “The bacteria were consistent gram-positive in every section.”
While the portal of entry for pathogenic Listeria sp. in cases of encephalitis is presumed to be the buccal mucosa, all other forms of disease cause by this pathogen in ruminants are presumed to develop through the lower gastrointestinal tract. Enteritis, the most common presentation in humans, has been rarely identified in sheep.5 Adult sheep may develop a marked enteritis with extensive necrosis of Peyer’s patches as well as necrohemorrhagic enteritis and abomasitis.5
Within the gut, bacteria will enter macrophages, often via M cells, whereupon they escape from phagolysosomes and are transported throughout the body. This bacteremia may develop into a listerial septicemia - septicemia occurs most often in immunosuppressed animals and pregnant animals, and its characteristic lesions are most often seen in the fetus. In the fetus, multiple foci of necrosis are seen in the liver and spleen and the organism is readily demonstrated within these lesions. Occasional massive outbreaks of septicemia involving pregnant mucinous have been described with clinically affected animals exhibiting fever and profuse diarrhea.5
The gravid uterus appears to be highly susceptible to infection during listerial septicemia in ruminants and many other species. Another pathogenic form of Listeria, L. ivanovii, has been recorded as a cause of abortion in cattle and sheep, albeit with less frequency than L. monocytogenes. In cases of listerial abortion, there are necrotic lesions within cotyledons as well as a diffuse intercondylar urinary placentitis characterized by a reddish brown exudate.5 The fetus is usually autolytic with evidence of septicemia as discussed previously.
Less common but documented listerial syndromes include iritis and keratoconjunctivitis in both cattle in sheep (likely complications of cranial nerve infection), as well as subclinical or clinical mastitis, both seen in silage-fed animals during winter.
Conference participants discussed the various virulence factors associated with Listeria, including internalin (initiates E-cadherin-facilitated entry into the cell), listeriolysin (a perforin which lyses the phagosomes of neutrophils and macrophages), and act A protein (which co-opts actin within the cytoskeleton to facilitate cell-to-cell transfer). Major differences between L. monocytogenes and H. somni, another neurotropic bacillus in ruminants, were identified as vasculitis in cases of H. somni, microabscess formation by L. monocytogenes, and L. monocytogenes’ preference for the brainstem as a result of transaxonal migration from the oral cavity along the cranial nerves.
Contributing Institution:
Massey University
Institute of Veterinary, Animal and Biomedical Sciences
http://ivabs.massey.ac.nz
http://vet-school.massey.ac.nz
References:
- Brugere-Picoux. Ovine listeriosis. Small Ruminant Res. 2008;76:12–20.
- Constable PD. Clinical examination of the ruminant nervous system. Vet Clin North Am Food Anim. 2004;20:185–214.
- Fentahun T, Fresebehat A. Listeriosis in small ruminants: A review. Advances in Biological Research. 2012;6:202-209.
- Hamon MA, Ribet D, Starvu F et al. Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol. 2012;20:36-368.
- Low JC, Donachie W. A review on Listeria monocytogenes and Listeriosis. Vet J. 1997;153:9-29.
- Maxie MG, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 5th ed. 1. Philadelphia, PA: Elsevier; 2007:282–457.
- Oevermann A, Di Palma S, Doherr MG, et al. Neuropathogenesis of naturally occurring encephalitis caused by Listeria monocytogenes in ruminants. Brain Pathol. 2010;20:378–390.
- Oevermann A, Zurbriggen A, Vandevelde M. Rhombencephalitis Caused by Listeria monocytogenes in Humans and Ruminants: A Zoonosis on the Rise? Interdiscip Perspect Infect Dis. 2010:2010:1-22
- Reuter R, Bowden M, Palmer M. Ovine listeriosis in south coastal Western Australia. Aust Vet J. 1989;66:223–224.