Wednesday Slide Conference, Conference 15, Case 1
Signalment:
Table egg layers (leghorn chickens), 27-weeks old.
History:
Flock of 15000 birds. 900 new birds were added to the flock on Feb 5, 2024. A sharp drop in egg production (14%) was?reported on February 15, 2024. Mortality begins on February 17 with 3+ birds dead per day until March 12, 2024. The cumulative mortality in the flock was 127 birds. The clinical signs included swollen face, eyelids, wattles, lacrimation, and mucoid nasal discharge.?
Gross Pathology:
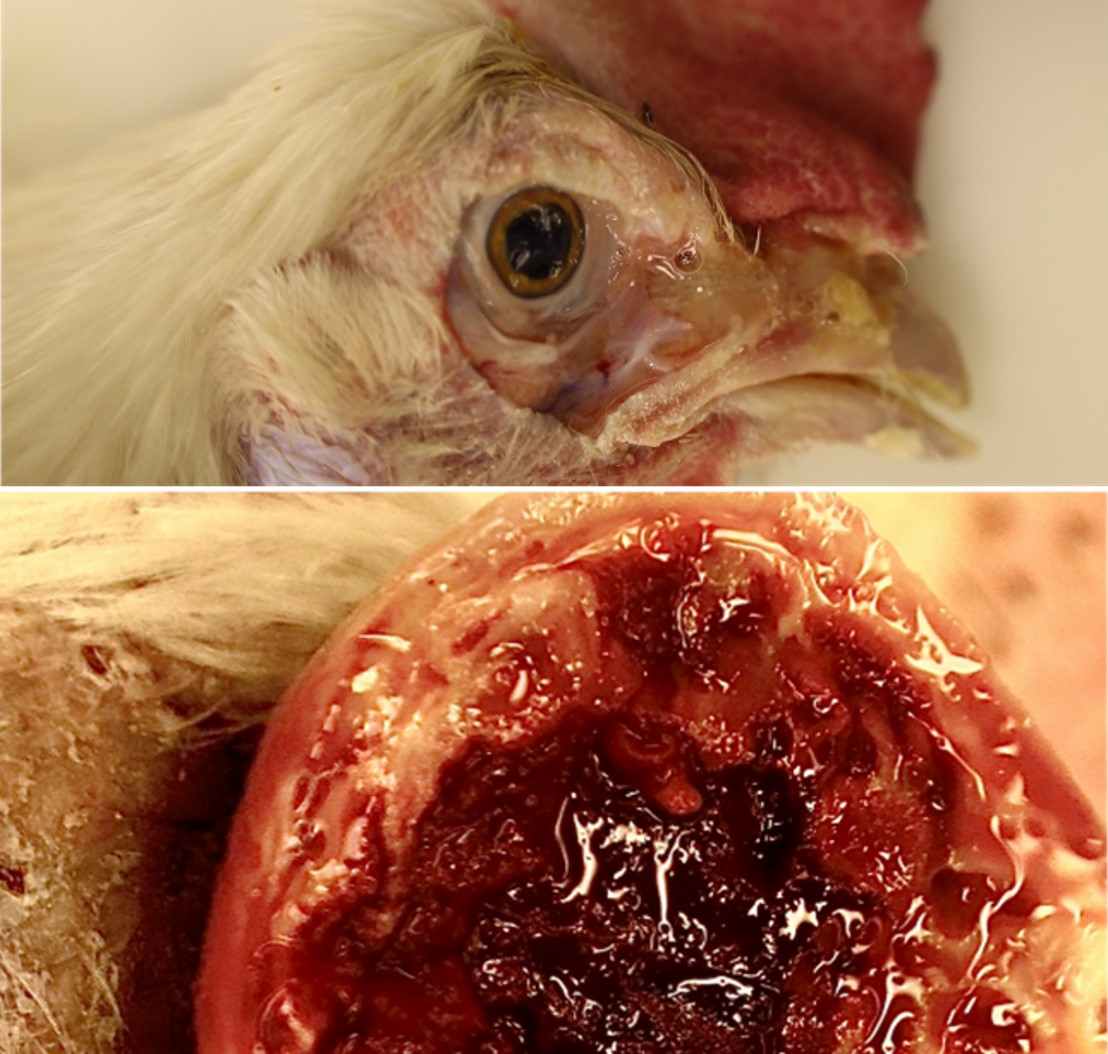
Two culled birds were submitted. The carcasses were fresh. The body condition was fair with apparent fat stores. The face, eyelids?and?wattles of the chickens were markedly swollen and red. The eyes were closed, and mucoid exudate expressed from the nasal sinuses. On reflecting skin, the subcutaneous tissue was markedly edematous. The cut surface of the wattles was dark red and soft in the center and surrounded by yellow-white tissue at the periphery. The thoracic and abdominal air sacs were yellowish and cloudy. There were multifocal, few, 1 mm, pale white foci in the liver parenchyma. The ovarian follicles were very small or had a few enlarging follicles (not in production). No significant gross findings in other organ systems.
Laboratory Results:
Avibacterium Paragallinarum?cultured from wattle and sinus swab.
Infectious Bronchitis Virus (IBV) and Mycoplasma spp. generic PCR- Low positive for IBV (CT= 32), negative for Mycoplasma spp.
Microscopic Description:
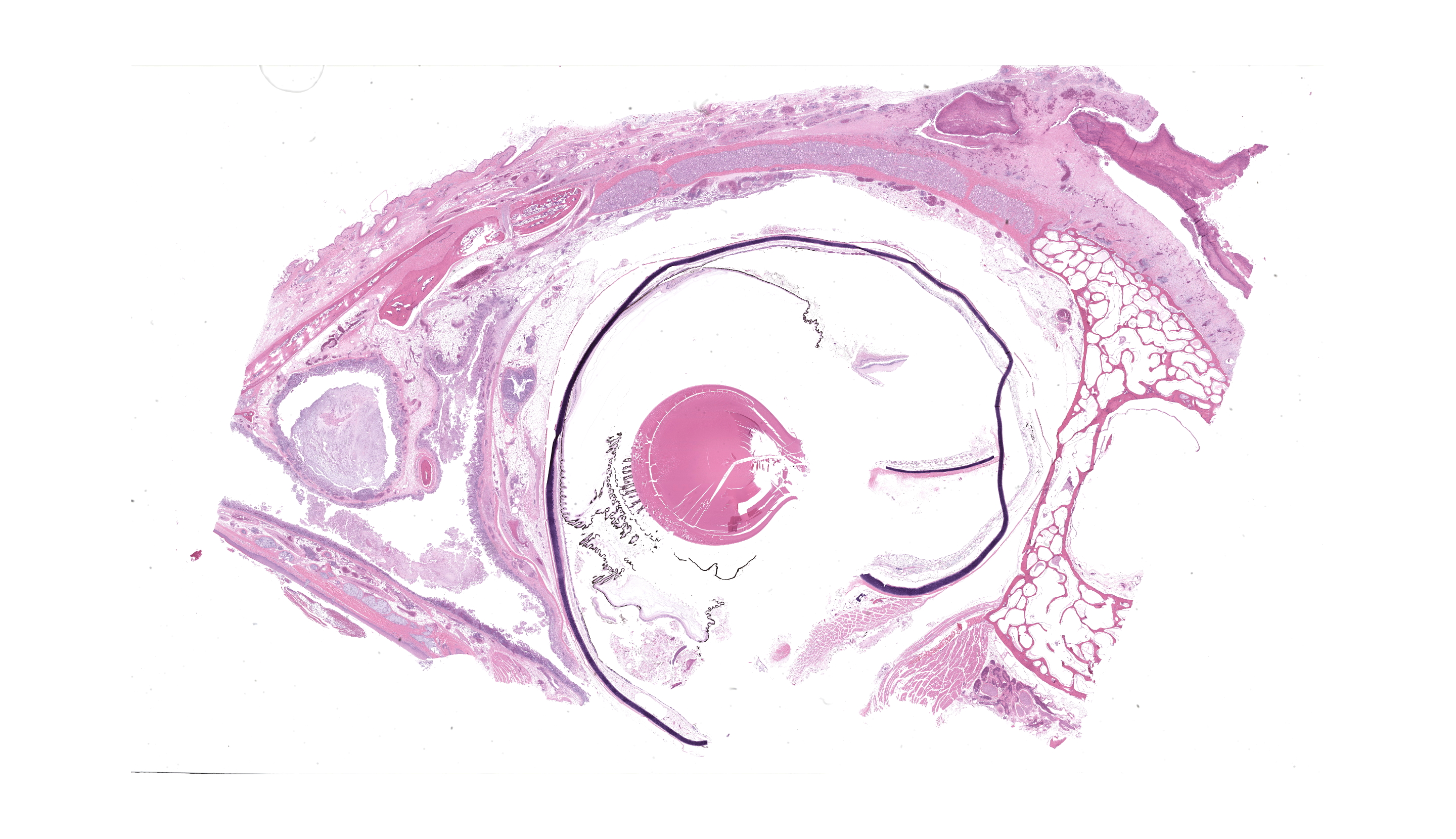
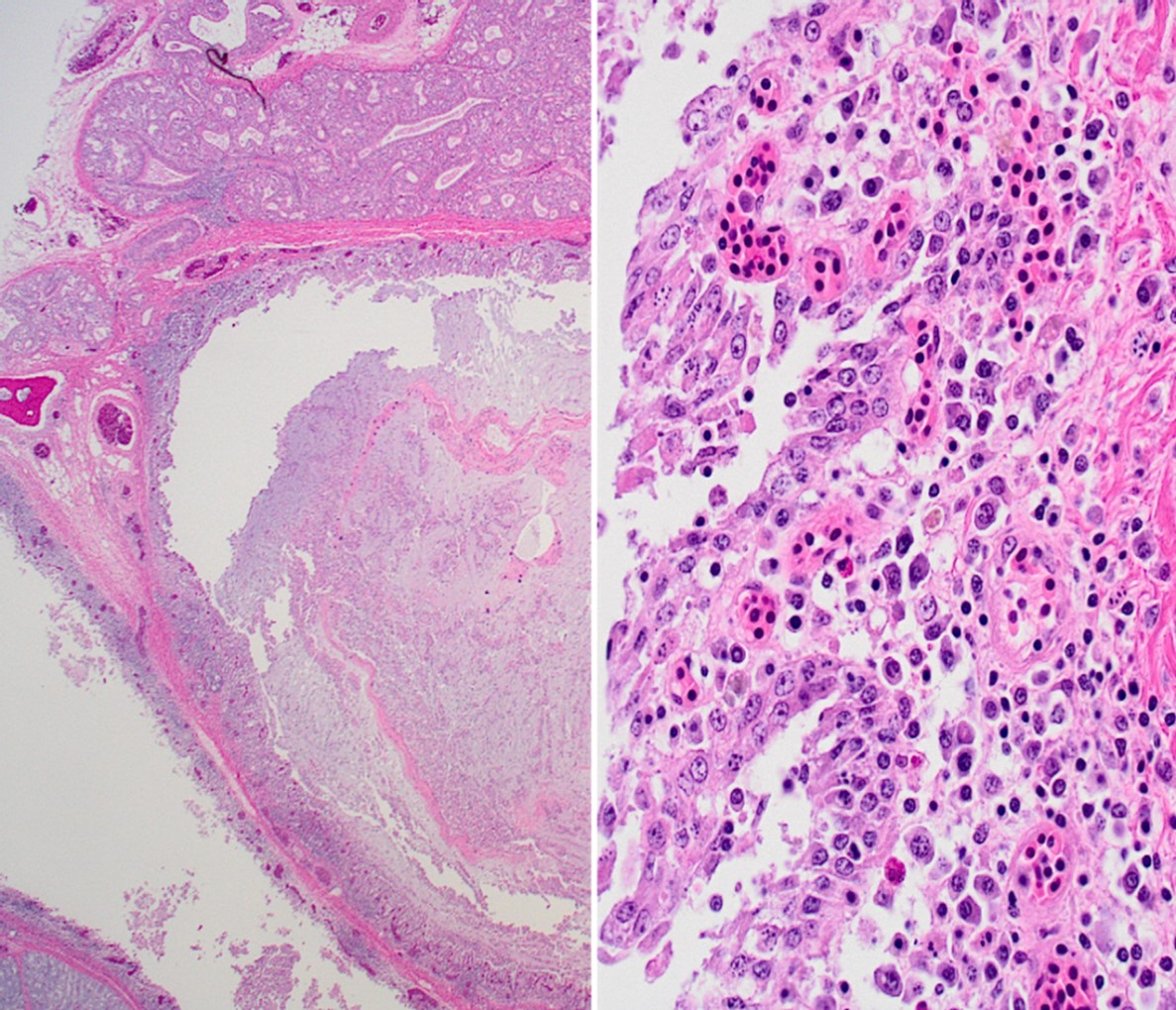
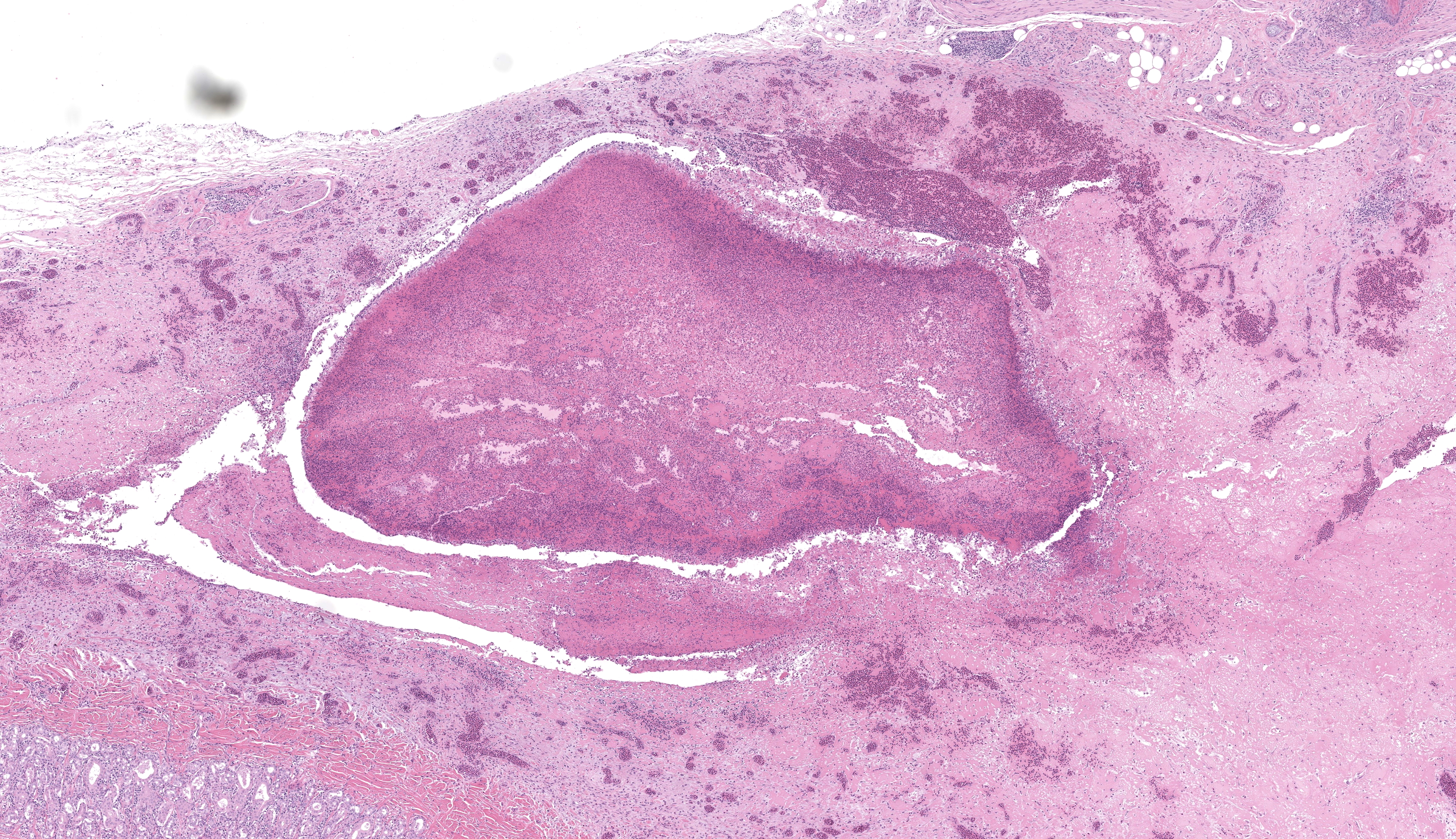
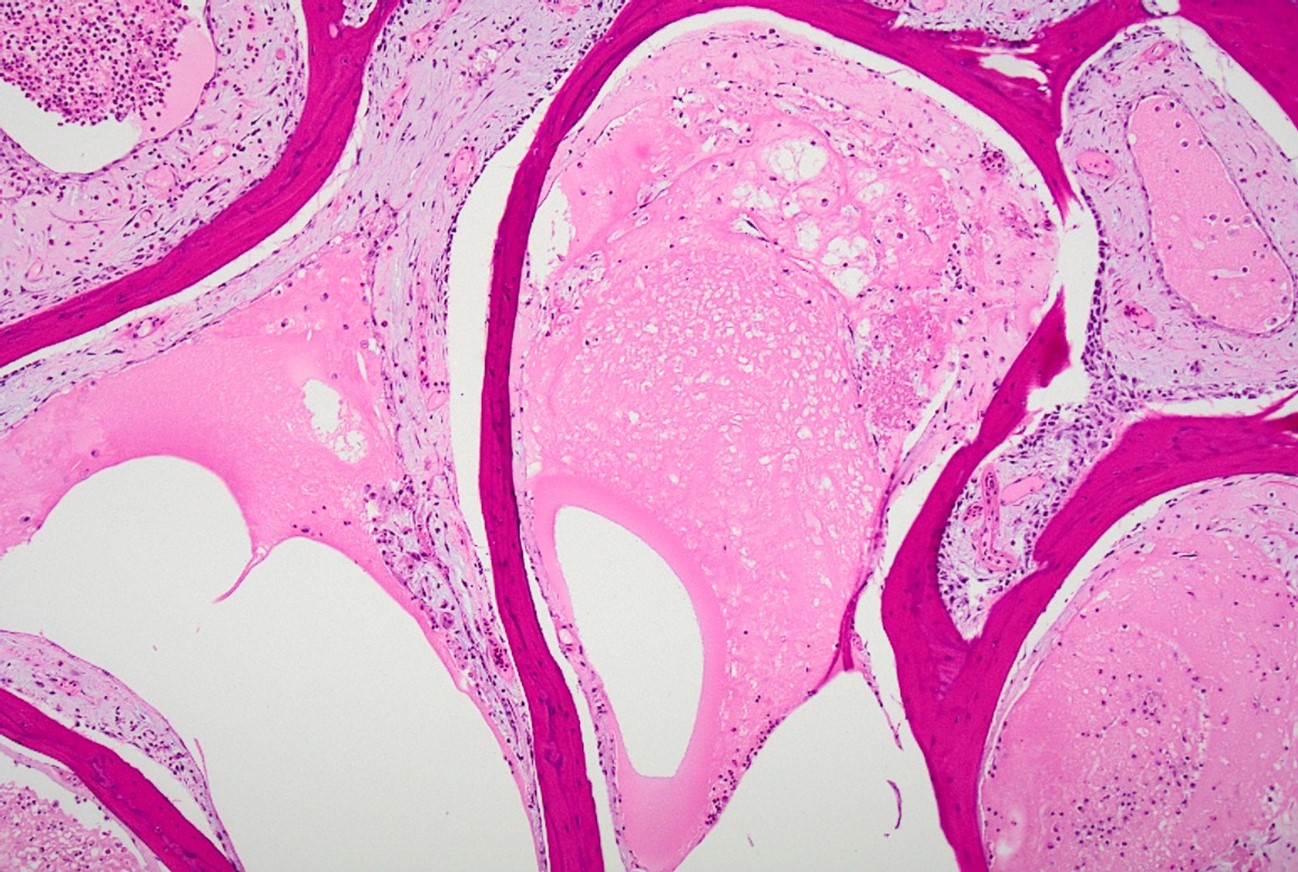
Wattle: Diffusely the center of the wattle was necrotic and hemorrhagic which is characterized by eosinophilic, cellular, and heterophilic debris mixed with pale eosinophilic fibrin, edema fluid, and hemorrhage. At the periphery was a layer of giant cells. The adjoining tissue was variably necrotic and?congested and there are multifocal perivascular and lymphocyte infiltrates. The infraorbital sinus was filled with pale blue homogenous mucous mixed with cellular debris, pale eosinophilic fibrin, and heterophils. The lining epithelium was multifocally sloughed and the lamina propria was multifocally congested, edematous, and infiltrated by lymphocytes, plasma cells, macrophages, and heterophils. A similar lesion is present in the lining epithelium of the nasal cavity. Lacrimal and harderian glands are moderately infiltrated by lymphocytes, plasma cells, and heterophils.? The air spaces in the calvarium are filled with eosinophilic fluid, mixed with fibrin, a?small number of heterophils, and sloughed lining epithelium. ?Other lesions included multifocal, hepatic necrosis and mild focal airscculitis, diffuse, lymphocytic and heterophilic, conjunctivitis, necro-hemorrhagic cellulitis.
Contributor’s Morphologic Diagnosis:
- Wattles: dermatitis, fibrino necrotizing and hemorrhagic, diffuse, marked, acute.
- Infraorbital sinus and nasal cavity: Sinusitis and rhinitis, lymphoplasmacytic and heterophilic, mucoid, marked, diffuse, acute.
- Cranial osteomyelitis, necrotizing, severe, multifocal, acute.
Contributor’s Comment:
Infectious coryza (IC) is an economically?important?disease of intensively raised commercial chickens?around the world. IC is an acute, sometimes chronic,?contagious,?upper respiratory tract disease that results in airsacculitis and condemnation in the broiler chickens in the processing plant and reduced egg production (up to 10-40%) in the breeders and layers.2 The disease?is spread?by close contact and droplets, fomites, or the introduction of carrier chickens into a closed flock. The incubation period is about 24 hours. The affected birds?in?uncomplicated?cases recover within 2 weeks.1 IC is caused by A. paragallinarum, which is a gram-negative, fastidious organism bacterium (previously, Hemophilus paragallinarum). It requires factor nicotinamide dinucleotide (NAD) for invitro growth, which?is often provided?by striking a satellite Staphylococcus aureus nurse colony on the plate. NAD- independent bacterial strains exist?which?sometimes pose a diagnostic challenge. In field conditions, it is often difficult to recover this bacterium, hence sending whole heads for bacterial culture is preferred if the veterinarians prefer to do necropsy in the field. A. paragallinarum can be categorized into 3 serovars or sub-serovars by hemagglutination inhibition tests 3,4 and/ or recently genotyping is used to categorize the bacteria with a high correlation with serovars, but the technique needs further studies.2 The?important?virulence factors of A. paragallinarum include hemagglutinins (HAs),?capsule, and RTX cytotoxin. HA is a 210 kDa protein coded by the HMTp210 gene. The HA protein confer hemagglutination, cell adhesion, and biofilm formation. The capsule protects the bacteria from bactericidal activity of immune cells.
In this case, two whole bodies were submitted with swollen face, eyelids, wattles, lacrimation, and mucoid nasal discharge.?Given the history of a sharp decline in egg production and upper respiratory signs, Avibacterium paragallinarum, Mycoplasma gallisepticum, and Mycoplasma synoviae were top differentials. An underlying Infectious bronchitis virus (IBV) was also speculated based on the history of bronchitis in the source flock from where the birds were sourced. Hemorrhagic wattles and nasal swabs were sent for bacterial culture and sensitivity, and trachea was sent for the detection of generic Mycoplasma and IBV PCR. The clinical signs were non-specific and can be observed with several other disease agents that can act as a primary pathogen or coexist concurrently with IC. The list includes Mycoplasma gallisepticum, M. synoviae, Ornithobacterium rhinotracheale, Gallibacterium anatis, Pasteurella multocida, and viral pathogens such?as Avian Metapneumovirus virus (aMPV), IBV and infectious laryngotracheitis virus (ILTV). In addition, poor management and ventilation can increase the severity of the disease.2 No other bacteria were cultured other than A. paragallinarum. Low level of IBV was detected in the tracheal samples and Mycoplasma spp. were negative on PCR. Owing to the low level of the virus, genotyping was not possible in this case. ILT was excluded based on histopathology and the aMPV was not tested in this case as it was not in Canada at the time of the current case. The birds were not laying in this case and were not treated and culled. In general, if laying birds get infected, they can be treated, though they can become carriers following treatment and recurrence is possible. It is recommended to cull the flock as soon as it is close to the cycle completion due to biosecurity risk to commercial poultry. The affected flocks can be depopulated if the disease is not reported in the region to avoid future?outbreaks. Inactivated bacterins are available to use in endemic areas to minimize the occurrence. Enhancing biosecurity and avoiding adding replacement birds to closed flocks are some?of the?strategies to reduce the occurrence of the disease.1 Prior testing before movement can be done. In this case, replacement birds were introduced into the current flock without any testing which resulted in the disease outbreak. On further investigation, the source flock had a similar disease outbreak in the past. This highlights the importance of strict biosecurity, and quarantining of newly introduced birds when not raised on the same site. While the disease is very prevalent in the US and other countries, there is not much information on the occurrence of IC in Canada.
Contributing Institution:
Pathology & Diagnostic Services | Diagnostic Services Unit (DSU) | Faculty of Veterinary Medicine | University of Calgary (ucalgary.ca).
Faculty of Veterinary Medicine | University of Calgary (ucalgary.ca)
JPC Diagnosis:
- Nasal cavity and infraorbital sinuses: Rhinitis and sinusitis, heterophilic and granulomatous, chronic, diffuse, severe, with luminal bacilli.
- Comb: Dermatitis, necrotizing and heterophilic, chronic, diffuse, severe.
- Harderian and conjunctival lacrimal glands: Dacryoadenitis, lymphoplasmacytic, chronic, diffuse, mild to moderate.
JPC Comment:
This conference’s moderator was Dr. Tom Cecere of Virginia-Maryland CVM who led conference participants through a broad cross-section of infectious agents and production animal species. We enjoyed reviewing this first slide given that it is a microcosm of the gross pathology that the contributor nicely demonstrates, though orientation required consideration of the glands, sinuses, and unaffected epithelium accordingly. Our Gram stain confirmed gram-negative coccobacilli present within the infraorbital sinus (consistent with Avibacterium) along with fewer gram-positive bacilli and cocci. We interpreted the large region of necrosis and cellulitis as arising within the comb of this bird; we did not see any bacteria within this region on H&E or Gram stains. We differed from the contributor on osteomyelitis as bony changes within our section were mild (perhaps reflecting variation in submitted slides) though we did capture additional inflammation within adjacent glands as a secondary change in this case.
The contributor lays out a good differential diagnosis list for this case. Participants noted multinucleated giant cells (especially within the overlying skin) that prompted a discussion of viral syncytia (e.g. ILTV) versus granulomatous inflammation and macrophage activation. Given the likely polymicrobial infection at play in these birds, consideration of relevant histologic features and ancillary diagnostics such as culture and PCR is prudent for the supporting pathologist.
Infectious coryza has also been reported on poultry farms and among smaller hobby flocks within the United States.5,6 In contrast to this case, more acute/fulminant disease may have more fibrinous exudate and infection can extend to the abdominal air sacs and/or the pericardial sac and cause pericarditis and/or periphepatitis.6 Complicated (polymicrobial) infections often lead to septicemia and marked flock mortality.5,6 Flock susceptibility to IC can reflect vaccine failure (poor preparation, timing/delivery failure) as well as lack of cross-protection between different circulating serovars; use of vaccines in laying birds, meat birds, and breeder flocks varies by facilty.6 Farm-farm transmission between poultry workers is a likely control factor as wild birds and insects have been shown to be ineffective vectors for IC.6
References:
- Blackall PJ, Soriano-Vargas E. Infectious Coryza. In: Swayne DE, Boulianne M, Lounge CM, McDougald LM, Nair V, Suarez DL, eds. Vol. 2, Diseases of Poultry. Wiley-Blackwell 2014:890–906.
- Gallardo RA, Da Silva AP, Egaña-Labrin S, et al. Infectious Coryza: Persistence, Genotyping, and Vaccine Testing. Avian Dis. 2020;64(2):157–165.
- Kume K, Sawata A, Nakai T, Matsumoto M. Serological classification of Haemophilus paragallinarum with a hemagglutinin system. J Clin Microbiol. 1983;17(6):958–964.
- Page LA. Haemophilus infections in chickens. I. Characteristics of 12 Haemophilus isolates recovered from diseased chickens. Am J Vet Res. 1962;23:85–95.
- Davison S, Tracy L, Kelly DJ, Bender SJ, Pierdon MK, Mills J, Barnhart DJ, Licciardello S, Anis EAM, Wallner-Pendleton E, Dunn P, Robinson C, Ladman B, Kuchipudi SV. Infectious Coryza in Pennsylvania. Avian Dis. 2024 Sep;68(3):175-182.
- Crispo M, Blackall P, Khan A, Shivaprasad HL, Clothier K, Sentíes-Cué CG, Cooper G, Blakey J, Pitesky M, Mountainspring G, Cutler G, Bickford A, Stoute S. Characterization of an Outbreak of Infectious Coryza (Avibacterium paragallinarum) in Commercial Chickens in Central California. Avian Dis. 2019 Sep 1;63(3):486-494.