Wednesday Slide Conference, Conference 20, Case 4
Signalment:
Juvenile, male, Megaptera novaeangliae, humpback whale.
History:
A juvenile male humpback whale, measuring 11 meters in length, was found stranded alive in the coast of southern Brazil. Due to its deteriorated health and unsuccessful attempts to return the animal to the ocean over two days, it was euthanized.
Gross Pathology:
At necropsy, the humpback whale was in poor body condition. Multiple marine crustaceans (Thoracica sp.) were adhered to the skin in the submandibular and ventral neck region. Upon internal examination, significant changes were noted in the liver. The hepatic capsula appeared opaque and whitish. The right hepatic lobe was moderately reduced in size and with multifocal and elevated areas, which upon sectioning corresponded to biliary ducts with thickened walls and dilated lumen. The lumen was filled with bile and with numerous trematodes measuring 1.5-4 cm in length, 0.3-0.4 cm in width, with flattened and leaf-shaped morphology, containing oral and ventral suckers. Morphological and molecular characterization classified these parasites as Brachycladium goliath. Inside the renal veins, a high number of nematodes of up to 30 cm in length, morphologically compatible with Crassicauda sp., were observed. Additional macroscopical findings included the multiple accessory spleens measuring from 1.5 to 5 cm in diameter. Furthermore, the small intestine showed multifocal to coalescent areas of hemorrhages (petechias and ecchymosis) and the mucosa was markedly red with cestodes measuring approximately 80 cm in length in the intestinal lumen. The gross examination of remaining organs was unremarkable.
Laboratory Results:
RT-Nested-PCR: positive for cetacean morbillivirus (CeMV).
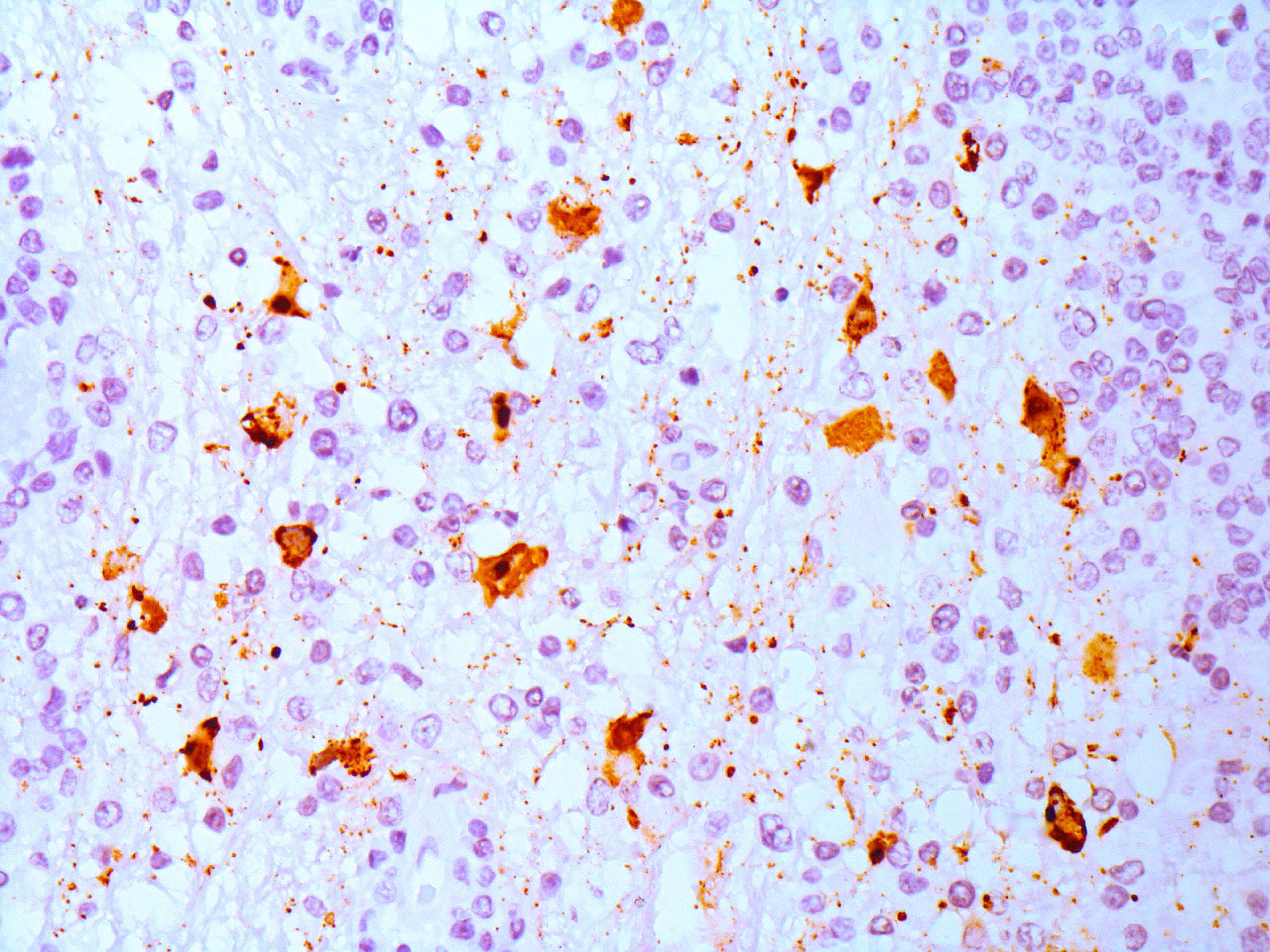
Immunohistochemistry (IHC): IHC anti-Morbillivirus (anti-canine distemper virus, monoclonal) revealed moderate cytoplasmic immunolabelling in astrocytes, neuronal cell bodies, and axons).
Microscopic Description:
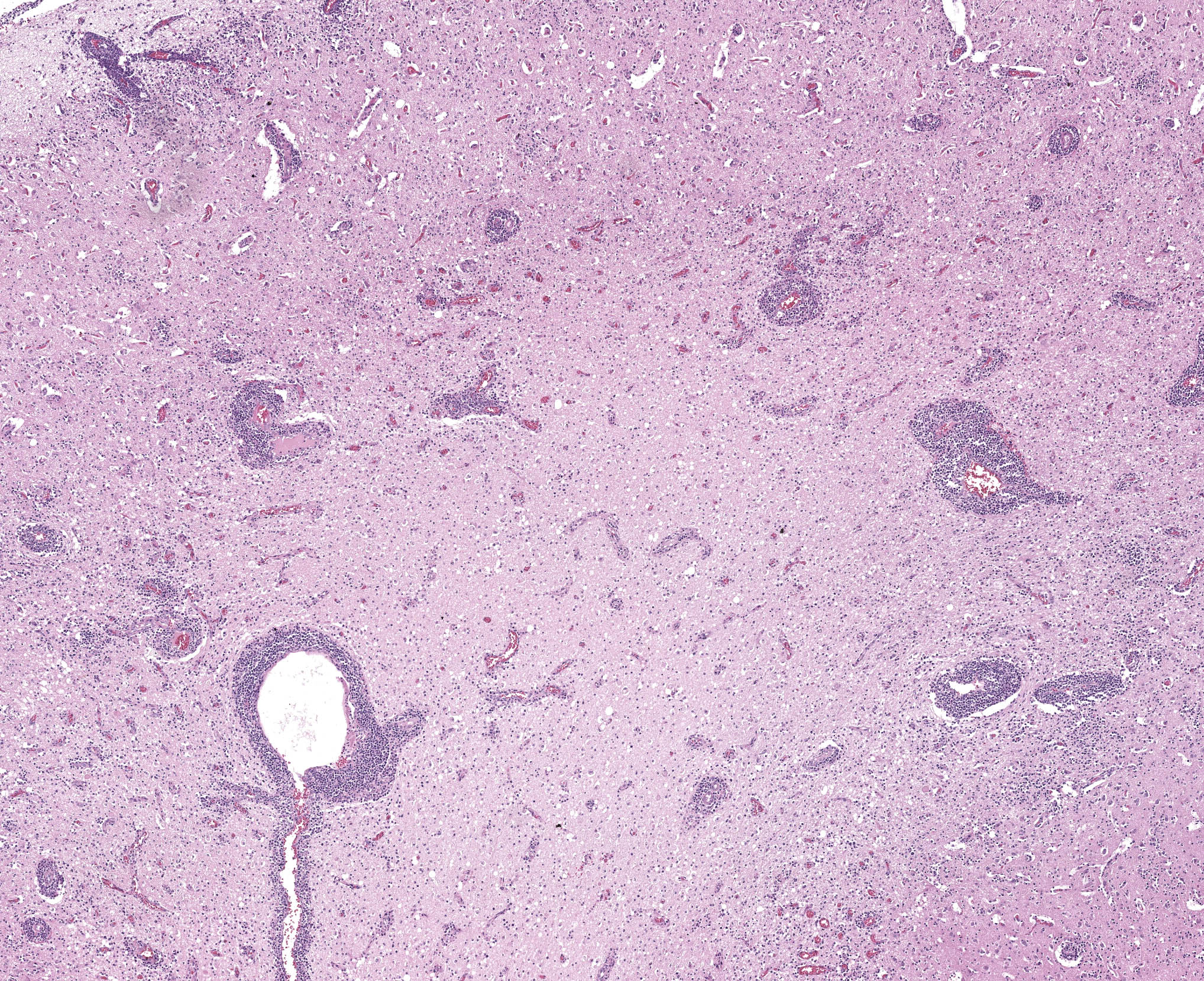
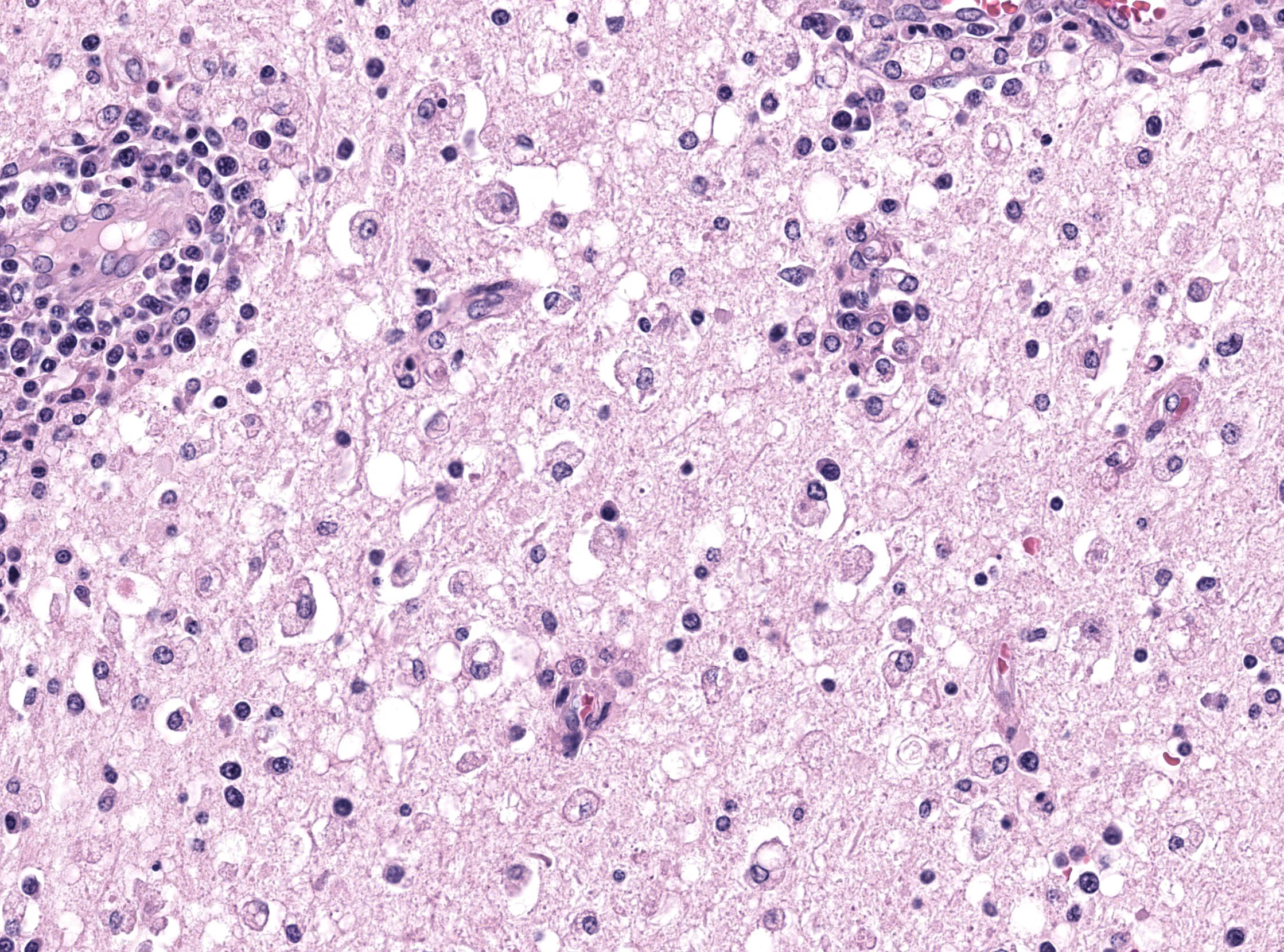
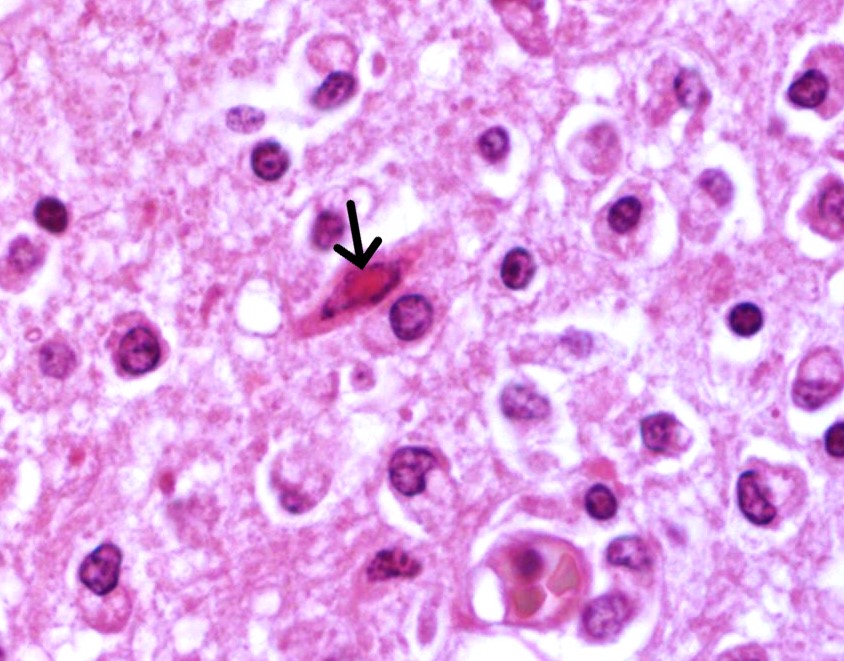
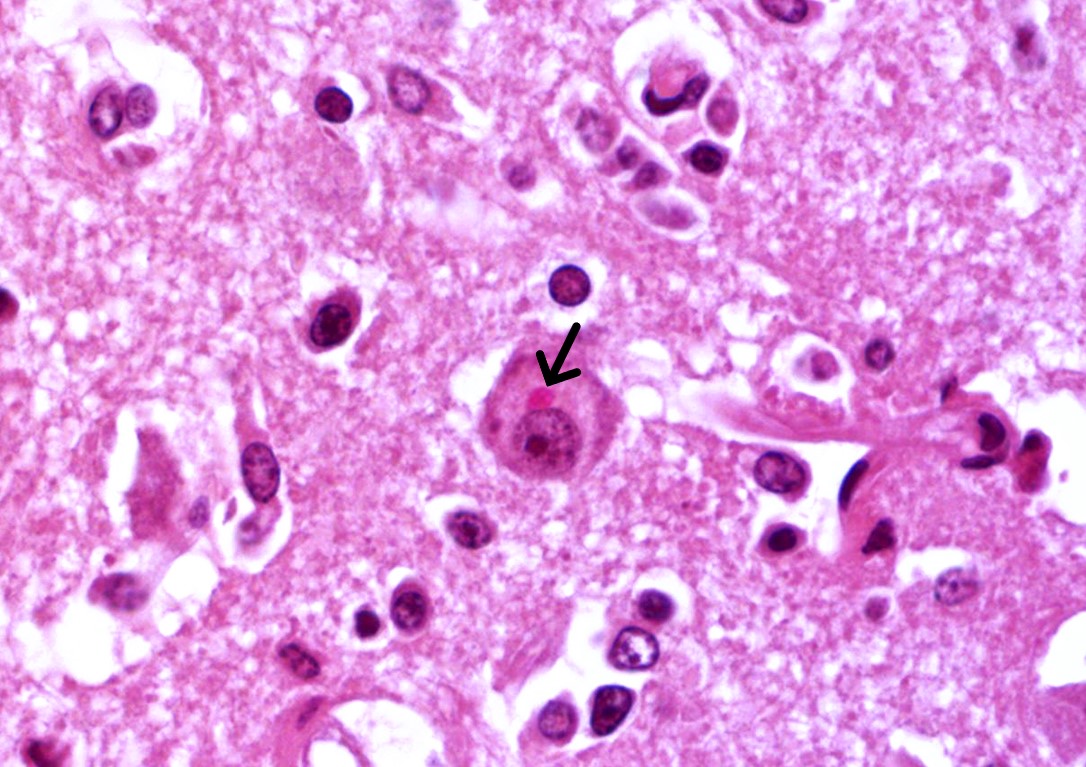
Brain: in the telencephalic cortex, mainly in the grey matter, there is marked and multifocal inflammatory infiltration of lymphocytes and plasma cells surrounding blood vessels (perivascular cuffings), frequently extending into the adjacent neuropil where moderate vacuolization is also frequent. Additionally, there are multifocal areas of moderate gliosis. Mild to moderate neuropil rarefaction (malacia) is evident in some areas, where there is neuronal necrosis, characterized by neurons with hypereosinophilic and retracted cytoplasm and absent or pyknotic nucleus. Moderate infiltration of Gitter cells and occasional axonal spheroids is also present. Occasional intracytoplasmic and intranuclear eosinophilic inclusion bodies are seen in neurons within affected areas. Similar lesions are also present in the brainstem and white matter of the cerebellum (slides not submitted).
Contributor’s Morphologic Diagnosis:
Brain: encephalitis, lymphoplasmocitic, multifocal, marked, subacute, with mild and multifocal malacia, Gitter cell inflammatory infiltration, neuronal necrosis and occasional intracytoplasmic and intranuclear eosinophilic inclusion bodies.
Contributor’s Comment:
Viruses from the genus Morbillivirus belong to the family Paramyxoviridae and order Mononegavirales. These are pleomorphic but frequently spherical, approximately 150 nm in diameter, RNA viruses with a nonsegmented and single-stranded linear genome.11 Morbilliviruses can affect different species of mammals, including humans. The measles virus (MeV) is a human pathogenic morbillivirus that causes measles, a disease with high rates of morbidity and significant childhood mortality. Rinderpest, also known as “cattle plague”, the peste des petits ruminants, and canine distemper are other notable diseases caused by morbilliviruses in mammals.16
Morbillivirus infections resulting in massive strandings have been reported in aquatic mammals in recent decades.3 The first description occurred in pinnipeds during 1988 and 1989 in northwest Europe, where nearly 18,000 harbor seals (Phoca vitulina) and hundreds of grey seals (Halichoerus grypus) died due to the infection by a morbillivirus identified as Phocine Distemper Virus (PDV).7,9 Meanwhile, in cetaceans, the first report of a morbillivirus infection was described in six harbor porpoises (Phocoena phocoena) in Ireland in late 1988. This infection was related to the Porpoise Morbillivirus (PMV), a strain from the Cetacean Morbillivirus (CeMV). A couple of years later, infections by this virus were reported in harbor porpoises from various countries across Europe.9,17 Since then, many strains of CeMV have been recorded causing stranding and deaths in odontocetes. However, reports in mysticetes are rare and include few cases in fin whales (Balaenoptera physalus)8 and more recently, in two humpback whales in Brazil.1
Morbilliviruses are known to be lymphotropic and epitheliotropic, and studies have indicated that the pathogenesis associated to the CeMV infection resembles what is commonly seen in other morbillivirus infections in animals and humans.10 When infected by CeMV, cetaceans can develop a marked interstitial pneumonia and/or non-suppurative encephalitis. Animals that survive this acute stage may acquire opportunistic infections due to the immunosuppression caused by the virus. Some animals may still have lesions of meningoencephalitis associated with demyelination. However, animals that clear out the systemic infection may develop a neurological disease with lesions restricted to the brain. In these cases, the virus is detected only in the CNS, and cytoplasmic or nuclear eosinophilic inclusion bodies are occasionally found in neurons, and astrocytes.3 In a similar way, in humans with measles, the MeV infection can lead to encephalitis with inclusion bodies, particularly in immunosuppressed individuals, or to panencephalitis in persistently infected children.5 Neurological involvement is common in carnivore morbilliviruses, as in the canine distemper, which can affect not only domestic dogs, but a broad range of domestic and wild carnivores.4 In dogs, a strong immune response typically clears the canine distemper virus (CDV) from the tissues, resulting in fully recovery. On the other hand, with a weak immune response, the virus can penetrate epithelial tissues and the CNS, leading to clinical signs that typically appear around 20 days post-infection.17 Neurologic signs can also develop later, due to a demyelination induced by the CDV.14
In this humpback whale, no macroscopic changes were seen during the evaluation of the encephalon, corroborating to previous studies on morbillivirus infections affecting the CNS of other species, such as dogs, in which neurological involvement is frequent.15 On histological examination, CeMV infection in cetaceans resembles the lesions described in humans and animals infected by morbilliviruses, primarily characterized by multiple mononuclear perivascular cuffings, microgliosis, neuronophagia and demyelination in the cerebral cortex. The white matter may also show areas of malacia. Intracytoplasmic and/or intranuclear eosinophilic inclusions bodies are frequently seen.6 Furthermore, when IHC anti-Morbillivirus is applied, a marked immunolabelling in neurons, axons, and dendrites, as well as in astrocytes and microglial cells is described in cetaceans.6 This is similar to what has been reported in dogs infected by CDV.15 In the humpback whale from the present case, immunolabeling was evident in astrocytes and neurons from the telencephalon, cerebellum and brainstem, corroborating the aforementioned studies.
Differential diagnoses for mononuclear meningoencephalitis in cetaceans are limited, but they include infections caused by herpesvirus13 and flaviviruses such as the West Nile Virus.12 However, the Cetacean Morbillivirus remains as the most common cause of viral meningoencephalitis in cetaceans.
Contributing Institution:
Faculdade de Veterinária
Universidade Federal do Rio Grande do Sul
Setor de Patologia Veterinária
http://www.ufrgs.br/patologia
JPC Diagnosis:
Cerebrum: Meningoencephalitis, lymphoplasmacytic and necrotizing, chronic, diffuse, moderate, with neuronal necrosis, Gitter cells, and rare intracytoplasmic and intranuclear viral inclusions.
JPC Comment:
The final case of this conference is a (rarely seen) well-preserved whale brain. Dr. Terio noted that the brain condition in this case is very good – that this animal was euthanized and immediately necropsied is helpful for appreciating the microscopic features that the contributor nicely captures. Difficulty in accessing the brain (especially in a stranding event) combined with carcass deterioration typically confound examination in wild whales.
Microscopic features of this case mirrored morbillivirus infection in other species. Perivascular cuffing, gliosis (glial nodules), and neuronal necrosis were readily apparent, though viral inclusions within the cytoplasm and nucleus were rare which Dr. Terio confirmed is usually the case in the brain. Inclusions may be more prominent within bronchiolar epithelium in animals with respiratory changes. Viral syncytial cells were also infrequent in our section, though they too are typically more prominent in other tissues. This can be a helpful ancillary finding in cases with marked lymphoid depletion and autolysis as syncytial cells may still be discernable.
Conference participants discussed potential comorbidities in cetacean morbillivirus cases. Coinfection with Brucella (e.g. B. ceti) also causes neuropathology18 and may be aided by concurrent lymphoid depletion arising from CeMV infection. Joint infection is another common sequalae of Brucella. Microscopically, there is marked infiltration of the meninges by lymphocytes; in acute cases, bacteria and neutrophils may be appreciated in Dr. Terio’s experience. Fungal infections are also abetted by lymphoid depletion, though there were no characteristic features in this case and we did not pursue any special stains.
References:
- Amorim DB, de Camargo LJ, Ribeiro PR, et al. Characterization of Cetacean Morbillivirus in Humpback Whales, Brazil. Emerg Infect Dis. 2024;30(6).
- Van Bressem MF, Duignan PJ, Banyard A, et al. Cetacean morbillivirus: Current knowledge and future directions. Viruses. 2014;6(12):5145–5181.
- Deem SL, Yates LH, Rebecca A, Montali, Richard J. Canine distemper in terrestrial carnivores: a review. J Zoo Wildl Med. 2000;31(4):441–451.
- Fisher DL, Defres S, Solomon T. Measles-induced encephalitis. Int J Med. 2015;108(3):177–182.
- Guardo G Di, Marruchella G, Agrimi U, Kennedy S. Morbillivirus Infections in Aquatic Mammals: A Brief Overview. J Vet Med. 2005;52:88-93.
- Heide-Jorgensen MP, Harkonen T, Dietz R, Thompson PM. Retrospective of the 1988 European seal epizootic. Dis Aquat Organ. 1992;13(1):37–62.
- Jo WK, Kruppa J, Habierski A, et al. Evolutionary evidence for multi-host transmission of cetacean morbillivirus. Emerg Microbes Infect. 2018;7(1):201.
- Kennedy S. Morbillivirus Infections in Aquatic Mammals. J Comp Path. 1998;119(3):201-225.
- Kennedy S, Smyth JA, Cush PF, Mcaliskey M, Mccullough SJ, Rima BK. Histopathologic and Immunocytochemical Studies of Distemper in Harbor Porpoises. Vet Pathol. 1991;28(1):1-7.
- Lamb RA, Parks GD. Paramyxoviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, eds. Fields Virology. 6th ed. Lippincott-Raven Press; 2013.
- Leger J, Wu G, Anderson M, Dalton L, Nilson E, Wang D. West Nile virus infection in killer whale, Texas, USA, 2007. Emerg Infect Dis. 2011;17(8):1531–1533.
- Sierra E, Fernández A, Fernández-Maldonado C, et al. Molecular Characterization of Herpesviral Encephalitis in Cetaceans: Correlation with Histopathological and Immunohistochemical Findings. Animals. 2022;12(9):1149.
- Skyes JE, Vandevelde, M. Canine Distemper Virus Infection. In: Skyes JE. Greene’s Infectious Diseases of the Dog and Cat. 5th ed. Elsevier; 2023.
- Sonne L, Oliveira EC, Pescador CA, et al. Achados patológicos e imuno-histoquímicos em cães infectados naturalmente pelo vírus da cinomose canina. Pesq Vet Bras. 2009;29(2):143–149.
- De Vries RD, Paul Duprex W, De Swart RL. Morbillivirus infections: An introduction. Viruses. 2015;7(2):699–706.
- Welsh MJ, Lyons C, Trudgett A, Rima BK, Mccullough SJ, Orvelp C. Characteristics of a cetacean morbillivirus isolated from a porpoise (Phocoena phocoena). Arch Virol. 1992;125(1-4):305-311.
- Winters KA, Mathes LE, Krakowka S, Olsen RG. Immunoglobulin class response to canine distemper virus in gnotobiotic dogs. Vet Immunol Immunopathol. 1983;5(2):209–215.
- Davison NJ, Brownlow A, Doeschate MT, Dale EJ, Foster G, Muchowski J, Perrett LL, Rocchi M, Whatmore AM, Dagleish MP. Neurobrucellosis due to Brucella ceti ST26 in Three Sowerby's Beaked Whales (Mesoplodon bidens). J Comp Pathol. 2021 Jan;182:1-8.